membrane permeability. Theory act
Membrane cleaning methods are based on different membrane permeability for the components of the gas mixture being cleaned.[ ...]
The selective permeability of membranes in the process of ultrafiltration is explained by a purely sieve separation mechanism - impurity particles that are larger than the size of the pores of the membrane do not pass through the membrane, only water is filtered through it.[ ...]
The selectivity and permeability of membranes must be considered in relation to the costs of obtaining oxygen-enriched air. Air separation costs depend on permeability, selectivity, geometric parameters of the membranes, module performance, cost of electricity and other factors. The cost of oxygen-enriched air is estimated in relation to equivalently pure oxygen, defined as the amount of pure oxygen required to mix with air (21% oxygen) to obtain the same amount and percentage of oxygen that is obtained in the gas separation process in question.[ ...]
Ultrafiltration is a membrane process for the separation of solutions whose osmotic pressure is low. This method is used in the separation of relatively high molecular weight substances, suspended particles, colloids. Ultrafiltration compared to reverse osmosis is a more efficient process, since high membrane permeability is achieved at a pressure of 0.2-1 MPa.[ ...]
Solid waste washing 434, 425 Membrane permeability 273 Straining 197 cl.[ ...]
Calcium ions have a great influence on membrane structures. The need for Ca2+ ions to stabilize membranes has long been pointed out. It was shown that the presence of Ca2+ ions in the surrounding solution is necessary for the formation of a surface membrane on an endoplasmic droplet isolated from interdistant cells of charophytes. The presence of Ca2+ at a concentration of 10 4 M promoted the formation of a surface membrane on the droplet, although not strong enough; a stronger membrane was formed at a concentration of 10-3 M and especially 10 2 M. When calcium ions are removed (for example, when treated with chelates or in the absence of Ca2 + in the medium), mucilage of root hairs is noted, and the permeability of membranes to other substances also increases. Ca2 + ions change and electrical properties of both artificial and natural membranes, reducing the charge density on the membrane surface.The lack of Ca leads to an increase in vacuolization, changes in chromosomes, rupture of ER membranes and other intracellular compartments.[ ...]
With an increase in the concentration of the separated solution, the permeability of the membranes decreases, and with an increase in pressure, it increases. After the purification process, a filtrate is obtained, depleted by 90-99.5 ° / o in the original compounds, and a concentrate sent for further processing.[ ...]
The response to acetylcholine and biogenic amines is to change the permeability of membranes to ions and/or induce the synthesis of second messengers. The presence of cAMP, cGMP, Ca2+, as well as synthesis and catabolism enzymes in the plant cell and its organelles, confirms the possibility of local mediation.[ ...]
So, under the action of microwave EMR (2.45 GHz), an increase in the cation permeability of erythrocyte membranes at room temperature was found, while in the absence of microwave EMR, a similar effect is observed only at a temperature of 37 °C.[ ...]
The metabolite funds are not evenly distributed throughout the cell, but separated by membranes and localized in separate compartments (chambers, compartments). The compartments of the metabolic funds of the cell are interconnected by transport flows. In accordance with the selective permeability of membranes, a spatial redistribution of intermediates and metabolic products occurs. For example, in a cell, the supply of ATP is maintained due to the "horizontal" links between the processes of photosynthetic and oxidative phosphorus formation.[ ...]
solution concentration. With an increase in the concentration of the separated solution, the permeability of the membranes decreases due to an increase in the osmotic pressure of the solvent and the effect of concentration polarization. With a Reynolds criterion value of 2000-3000, concentration polarization is practically absent, however, turbulization of the solution is associated with its multiple recirculation, i.e., with energy costs, and leads to the accumulation of suspended particles in the solution and the appearance of biological fouling.[ ...]
A decrease in water temperature, leading to cooling of fish, also leads to an increase in the permeability of membranes, which lose their ability to maintain ionic gradients. In this case, the conjugation of enzymatic reactions is disturbed, ion pumps stop working, the work of the central and peripheral nervous system, the work of the cardiorespiratory apparatus is inhibited, which ultimately can lead to the development of hypoxia. During overheating or cooling of fish resulting from a sharp change in temperature in a limited time, a certain role belongs to osmotic stress due to a violation of the body's ability to maintain a certain concentration of ions and proteins in the blood. So, for example, a decrease in temperature from 25 to 11 ° C causes in tilapia contained in fresh water, the development of a coma, accompanied by a decrease in the concentration of sodium and chlorine ions and total blood protein. According to the authors, the death of fish occurs due to the development of osmoregulatory collapse and inhibition of kidney function. indirect confirmation This assumption may be the prevention of thermal coma in fish kept in dilute sea water, which is consistent with earlier observations of an increase in thermal resistance of fish due to the addition of sodium, calcium and magnesium ions to the water. However, it should be borne in mind that the causes of fish death at elevated or low temperatures are different and depend on the duration and intensity of the temperature effect.[ ...]
pH value. A change in the initial pH usually results in a decrease in membrane permeability. The effect of pH on membrane selectivity is small. Volatile acids are poorly retained by membranes, therefore, preliminary neutralization of volatile acids increases the selectivity of the separation process.[ ...]
At high salt concentrations in a three-chamber electrodialyzer with inert membranes, the maximum current efficiency does not exceed 20%.[ ...]
Received positive results wastewater treatment from OP-7 by reverse osmosis at a pressure of 5 MPa. Membrane permeability was 5-20.8 l/(m2-h) at a concentration of OP-7 in the filtrate of 1-18 mg/l.[ ...]
Surfactants (alkyl sulfates) stimulate the reproduction of bacteria to the greatest extent. In addition, surfactants, by changing the permeability of the membranes of living cells (S. S. Stroev, 1965, etc.), may contribute to better digestibility of nutrients contained in water by microbes.[ ...]
The nature of the solute has a certain effect on selectivity and, to a lesser extent, on membrane permeability. This influence is that inorganic substances are retained by membranes better than organic ones with the same molecular weight; among related compounds, for example, homologues, substances with a higher molecular weight are better retained; substances that form bonds with the membrane, for example, hydrogen, are retained by the membrane the better, the less strong this bond is; the selectivity of the retention of macromolecular compounds by ultrafiltration is the greater, the greater the molecular weight of the solute.[ ...]
Cellulose acetate membranes can operate in the pH range of 4.5-7, and those made of chemically resistant polymers can operate at pH 1-14. The permeability of the membranes is chosen to allow the passage of water, soluble salts and retain oils. The pore size in membranes is usually in the range of 2.5-10 nm. The plant is equipped with auxiliary pipelines for flushing the membranes with filtrate or demineralized water, equipped with instrumentation and automatic devices.[ ...]
With a significant decrease in the intracellular potential difference to a certain threshold level, a sharp change in membrane permeability and reversal (reversion) of ion fluxes are observed. Calcium ions from the external environment surrounding the cell enter it, while chloride ions and potassium ions leave the cell into the bathing solution.[ ...]
Tolerance is associated with internal factors and includes such metabolic processes as selective uptake of ions, reduced membrane permeability, immobilization of ions in certain parts of plants, removal of ions from metabolic processes through the formation of a reserve in insoluble forms in various organs, adaptation to the replacement of a physiological element with a toxic one in the enzyme, removal of ions from plants when washed out through the leaves, juice excretion, shedding of leaves, excretion through the roots. Tolerant plants can be stimulated at elevated concentrations of metals, which indicates their physiological need for excess. Separate types plants are capable of accumulating a significant amount of heavy metals without visible signs of oppression. Other plants do not have this ability (see table[ ...]
Pressure is one of the main factors that determine the performance of reverse osmosis plants. The performance of the membranes increases with an increase in excess pressure. However, starting from a certain pressure, the permeability of the membranes decreases due to the compaction of the polymeric material of the membrane.[ ...]
It has also been established that low ([ ...]
Since hemicellulose polysaccharides have a number average molecular weight of no more than 30,000, the use of conventional osmometry is difficult due to the permeability of membranes for low molecular weight fractions. Hill's method of vapor phase osmometry has a number of advantages over other methods. This method is based on measuring the difference between the vapor pressure of a solution and a solvent and is as follows. A drop of solution and a drop of solvent are placed on two thermocouple junctions and kept in an atmosphere saturated with pure solvent vapors. Due to reduced pressure solution vapor, part of the vapor will condense on the drop of solution, raising the temperature of the drop and the thermocouple. The resulting electromotive force is measured with a galvanometer. The upper limit of the measured value of the molecular weight is about 20,000, the measurement accuracy is 1%.[ ...]
Finally, the membranes of the endoplasmic reticulum are the surfaces along which biocurrents propagate, which are signals that change the selective permeability of membranes and thereby the activity of enzymes. Thanks to this, some chemical reactions are set in motion, others are inhibited - the metabolism is subject to regulation and proceeds in a coordinated manner.[ ...]
The plasmalemma regulates the entry of substances into the cell and their exit from it, ensures the selective penetration of substances into and out of the cell. The rate of penetration through the membrane of different substances is different. Water and gaseous substances penetrate well through it. Fat-soluble substances also easily penetrate, probably due to the fact that it has a lipid layer. It is assumed that the lipid layer of the membrane is permeated with pores. This allows substances that are insoluble in fats to pass through the membrane. The pores carry an electrical charge, so the penetration of ions through them is not completely free. Under certain conditions, the charge of the pores changes, and this regulates the permeability of membranes for ions. However, the membrane is not equally permeable for different ions with the same charge, and for different uncharged molecules of similar sizes. This shows the most important property of the membrane - the selectivity of its permeability: for some molecules and ions, it is better permeable, for others worse.[ ...]
Currently, the mechanism of action of mediators in animal and plant cells, which is based on the regulation of ion fluxes, is generally recognized. Changes in membrane potentials are due to shifts in the ion permeability of membranes by opening or closing ion channels. This phenomenon is associated with the mechanisms of occurrence and propagation of AP in animal and plant cells. In animal cells, these are N7K+ channels controlled by acetylcholine and Ca2+ channels, more often dependent on biogenic amines. In plant cells, the occurrence and spread of AP is associated with calcium, potassium and chloride channels.[ ...]
With greater reproducibility and stability, a stable flow of gases and vapors can be obtained by methods based on the diffusion of gases or liquid vapors through a capillary (Fig. 10) or a permeable membrane (Fig. 11) into the diluent gas stream. In such methods, an equilibrium is observed between the gas phase and the adsorbing surfaces of the equipment, which ensures the stability of the microflow.[ ...]
An increase in temperature leads to a decrease in the viscosity and density of the solution and, simultaneously, to an increase in its osmotic pressure. Reducing the viscosity and density of the solution increases the permeability of membranes, and an increase in osmotic pressure reduces the driving force of the process and reduces the permeability.[ ...]
In any living system, there is a REB, and it would be surprising if it were not. This would mean absolute equality of electrolyte concentrations in all cells, organs, external solutions, or complete coincidence of membrane permeability to all cations and anions.[ ...]
In experiment 6, similar to experiment 1, the amount of released potassium and water-soluble organic matter was determined at different concentrations of atrazine. Judging by the results obtained, it can be said that atrazine does not increase the permeability of membranes for low molecular weight organic substances and increases for potassium. This effect was proportional to the concentration of atrazine.[ ...]
When examining persons exposed to low-level radiation during work (for example, radiologists and technicians working with X-rays, the doses of which were measured by individual dosimeters) using the method of labeled atoms, blood tests were performed on the permeability of erythrocyte membranes during the passage of monovalent cations. It was found that the permeability of erythrocyte membranes in irradiated individuals is significantly higher than in those who were not irradiated. In addition, the dependence plot made it possible to establish a rapid increase in permeability at low irradiation; at high doses, the curve becomes flat, similar to Stokke's observation in animal studies (see Fig. XIV-3). These data are consistent with the results obtained by Petkau.[ ...]
When desalination of saline wastewater by hyperfiltration through semi-permeable membranes, the main parameters - the concentration of dissolved substances in the concentrate and filtrate must be determined per unit width of the membrane at a given length, separating capacity, membrane permeability coefficient, pressure, flow rates of source water, filtrate and concentrate.[ .. .]
The possibility of such adaptation is due to the dependence of thermodynamic, chemical, and kinetic constants on temperature. This dependence, in general, determines the direction and speed chemical reactions, conformational transitions of biological maodomolecules, phase transitions of lipids, changes in membrane permeability and other processes, the functioning of which ensures the vital activity of organisms at elevated temperatures.[ ...]
All this is only the first steps in the field of application of magnetic water in medicine. However, the already available information indicates the prospects for the use of magnetization of water systems in this area. A number of medical manifestations are possibly (hypothetically) related to the fact that the magnetization of water systems increases the permeability of membranes.[ ...]
It has been established that polymer films produced by the industry for ultrafiltration, ion exchange, as well as membranes made of collodion, gelatin, cellulose and other materials, have good selectivity, but low permeability (0.4 l/m h at a pressure of 40 am). Membranes prepared according to a special prescription from a mixture of cellulose acetate, acetone, water, magnesium perchlorate and hydrochloric acid (respectively 22.2; 66.7; 10.0; 1.1 and 0.1 weight percent) make it possible to desalinate water from 5, 25 to 0.05% NaCl and have a permeability of 8.5-18.7 l!m2 ■ h at an operating pressure of 100-140 am, their service life is at least 6 months. Electron microscopic studies of these membranes, since, according to preliminary calculations 1192], reverse osmosis can become competitive with other methods of water desalination with an increase in membrane permeability up to 5 m31 mg per day.[ ...]
The resting potential of the cell wall. The cell wall (shell) has a negative surface charge. The presence of this charge gives the cell wall distinct cation-exchange properties. The cell wall is characterized by predominant selectivity for Ca2+ ions, which plays an important role in the regulation of membrane permeability with respect to K and Na+ ions.[ ...]
Thus, the noted effects indicate that the culture fluid of the micromycete Fusarium oxysporum contains, in addition to fusaric acid, other components with high biological activity. The degree of pathogenicity of various isolates of phytopathogenic fungi can be assessed on the basis of determining changes in the permeability of plant cell membranes to ammonia.[ ...]
As a result, the formation of ATP is reduced or stopped, which leads to the suppression of processes that depend on the energy of respiration. The structure and selective permeability of membranes are also disturbed, which requires the expenditure of respiratory energy to maintain. These changes lead to a decrease in the ability of cells to absorb and retain water.[ ...]
On the other hand, the stabilization of the spatial structure of the protein and other biopolymers is carried out to a large extent due to the interaction: biopolymer - water. The water-protein-nucleic complex is considered to be the basis for the functioning of living systems, since only in the presence of these three components is the normal functioning of membranes possible. The selective permeability of membranes depends on the state of the water. Extrapolating the cluster model of water to biological systems, it can be shown that when the cluster is destroyed in certain areas of the membrane, a path for preferential transport opens. Structureless water, for example, prevents the behavior of protons near the membrane, while protons propagate rapidly along a structured framework.[ ...]
A scheme for continuous gas analysis using an ion-selective electrode is described, which can be used to determine the content of NH3, HCl, and HP in gases. In the review of the work of the NBS of the USA, among other methods of certification of reference gases (mixtures), the method of certification using ion-selective electrodes for gases of NSI and NR is also indicated. Of all the designs of ion-selective electrodes, the following is usually used: an ion-selective membrane separates two solutions - internal and external (tested). For electrical contact, an auxiliary electrode is placed in the internal solution, reversible to the ions of the internal solution, the activity of which is constant, as a result of which the potential is also constant. A potential difference arises on the inner and outer surfaces of the membrane, which depends on the difference in the activity of ions in the external and internal solutions. The theory of the appearance of the membrane potential is described in the work. Basically, the appearance of the potential is explained by the permeability of membranes either only for cations (cation-selective) or only for anions (anion-selective).
The bimolecular layer of phospholipids is the basis of any cell membrane. Its continuity determines the barrier and mechanical properties of the cell. In the process of life, the continuity of the bilayer can be broken with the formation of structural defects such as through hydrophilic pores. It is quite natural to expect this. All functions of the cell membrane are changed, including permeability and stability.
Phospholipids, which form the basis of cell membranes, are liquid crystals. As in any real crystal, a film of phospholipids can contain defects, in the place of which the main events of structural rearrangements develop. The types of defects are diverse, but the most natural for a bilayer is a defect of the type of a through hydrophilic pore.
In the bimolecular lipid film of the cell membrane, pores appear, if purely mechanical damage is excluded, as a result of thermal fluctuations of the bilayer surface, electrical breakdown, film freezing, the action of surfactants, osmotic pressure, lipid peroxidation, etc. One of the most typical and well-studied examples of destabilization of biological membranes - hemolysis of erythrocytes. This phenomenon includes, at the initial stage, the swelling of cells in a hypotonic medium as a result of the action of osmotic pressure forces. During cell swelling, the membrane is stretched, which causes an increase in membrane tension. At a certain threshold level of tension, hydrophilic lipid pores appear. The pore sizes are sufficient for the release of hemoglobin molecules and low molecular weight substances. The release of substances is accompanied, in turn, by a decrease in the difference in osmotic pressure, while the tension of the membrane decreases and the pores heal. Cytoskeletal proteins allow the erythrocyte to maintain its shape, thus forming the so-called shadow of the erythrocyte. The shadow retains osmotic activity and thus the process of destabilization acquires a cyclic character. Complete mechanical destruction of the cell like a soap bubble does not occur in this case. In the absence of the cytoskeleton or its insufficient development, the mechanical strength of the cell is entirely determined by the fate of the lipid pores. If the pore has a size less than the critical one, then it heals. Otherwise, the unlimited growth of the pore leads to the destruction of the membrane.
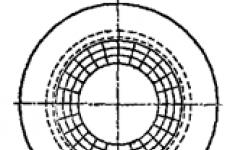
Critical pore model. Consider the lipid pore model (Fig. 15). We assume that the lateral surface of the pore has the shape of a circular cylinder. Moreover, suppose that the lateral surface of the cylinder is curved and has a radius of curvature h/2. The pore radius is r. As can be seen, the lipid bilayer as a whole is flat, and the pore has two radii of curvature h/2 and r. The curvature of the surface at the lipid-water interface is accompanied by the appearance of an additional pressure, called Laplacian and equal to
P = 2s 1 /r
where s 1 is the interfacial tension inside the pore, r is the radius of curvature.
Fig.15. The structure of the hydrophilic lipid pore: h is the thickness of the lipid bilayer; h/2 - wall curvature radius; r - pore radius.
In the model under consideration, there are two such radii (h/2 and r) and, consequently, two pressures. One of them P (h/2) contributes to the expansion, and the other P (r) - to the compression of the pore. Further fate pores depends on the ratio of these two pressures. If P (h / 2) > P (r), the time will expand, and if P (h / 2) is less than P (r), then the time will leak.
Consider the energy of the pore. As stated above, two opposite forces act at the pore boundary, one of which, the edge linear tension of the pore perimeter, promotes pore growth, and the second force, the surface tension of the bilayer, causes pore compression. The edge energy of the pore is proportional to the first power of the radius and increases the total energy, the surface tension energy is proportional to the square of the radius and reduces the total energy. As a result, the total energy E (r) is equal to
E(r) = 2pr 2 s
where the first term is determined by the energy of the pore edge with linear tension g, and the second term is determined by the surface tension energy s.
Taking into account the instability of the equilibrium, it can be argued that the appearance of pores with r>r* (r*=g/s) will leak and the stability of the membrane will remain. This is the criterion for the stability of a lipid bilayer membrane.
Electrical breakdown of the membrane. Biological membranes are under the action of an electric field of high intensity, created by diffusion of ions through the membrane and electrogenic ion pumps. The potential difference between the cytoplasm and the extracellular medium reaches about 0.1 V, the membrane thickness does not exceed 10 nm, which means that the field strength is 10 7 V/m. The membrane is a more perfect electrical insulator than many liquid insulators used in engineering. The membrane potential in a living cell can reach 0.2 V (freshwater algae, bacteria, energized mitochondria). in excitable nerves and muscle cells there is a short-term repolarization of the membrane with an increase in the amplitude of the potential. However, breakdown of the cell membrane by its own membrane potential is unlikely. At the same time, the increase in the membrane potential as a result of exposure to an external electric field can reach a value that exceeds the threshold for electrical breakdown. In this case, structural defects such as through lipid pores appear. The developed technique for electrical breakdown of cell membranes is called electroporation and is widely used in biotechnology.
In physics under electrical breakdown understand a sharp increase in the strength of an electric current in an initially weakly conductive medium. In a living cell, such a medium is the bimolecular lipid layer. For a lipid bilayer in the liquid-crystal state, the value of the membrane potential cannot be less than 0.23 V. The stability of bilayer membranes is determined by the probability of the appearance of pores of critical radius. Obviously, any factor that reduces the height of the energy barrier will increase this probability. These factors include a decrease in the pore edge energy y, an increase in the surface tension, and an increase in the membrane potential. Electrical breakdown is accompanied by the appearance of a wide range of lipid pores of various radii, including the radii of ion-selective protein channels. At present, the method of exposure to an external electric field is one of the main ones in modern biotechnology. It is known to be used to increase the porosity of membranes (electroporation), the introduction of DNA (electrotransfection), the release of cells from large molecules (electropermeabilization), cell fusion (electrofusion).
Temperature phase transition of membrane lipids. Freezing of the lipid bilayer as a result of the phase transition from the liquid crystal state to the gel is accompanied by the appearance of lipid pores. It is obvious that, as in the case of electrical breakdown, the fate of the membrane will be determined by the ratio of the radii of the formed pores and the critical pores for a given state of the bilayer.
The critical pore radius in the gel state is much smaller than in the liquid crystal state and does not exceed 2 nm in absolute value. Preservation of the long-term stability of the lipid bilayer in the gel state indicates that the existing pores and the pores that appear during the phase transition are smaller than 2 nm. Freezing of membrane lipids during the phase transition is equivalent to an electrical breakdown of the membrane by an external electric field of 0.5 V. Any mechanical, physical or chemical effect that affects the surface tension of the lipid bilayer is a risk factor in the stabilization of pore-containing membranes. The development of this approach makes it possible to obtain a quantitative answer to the question, important for biology, of the probability of destruction or healing of membranes under typical stress conditions of a living cell.
The critical radius of pores in membranes that are in the liquid-crystal state in the absence of external influences reaches 9 nm. This value is so significant that the probability of mechanical rupture of cell membranes under physiological conditions is very small. The rupture of a membrane in this state is possible only when the pore acquires dimensions commensurate with the thickness of the membrane. Experience shows that the complete destruction of the lipid bilayer is possible only with rough mechanical manipulations or irreversible electrical breakdown of lipids (IGS), gel state (gel), electrical breakdown (ep), when the gel state is combined with electrical breakdown (gel + ep) .
The sizes of critical pores for the lipid bilayer in the liquid crystal state (9 nm) are much larger than the sizes of real pores. Membranes in various stress states have a significant margin of safety, the effect of electrical breakdown and freezing of the bilayer is additive. Such a result can be expected, therefore, with other combinations of physical and chemical influences. Stress impact thus, irrespective of its physicochemical nature, can be quantified and its result predicted within the considered model. Model of pore formation during phase transition. An independent estimate of the pore size can be obtained by studying the proposed V.F. Antonov and collaborators of the model of pore formation. During the phase transition from the liquid-crystal state to the gel, according to X-ray diffraction analysis, there is a change in the thickness of the bilayer and the area per lipid molecule. Taking into account the cooperativity of the phase transition, it can be assumed that the molecules in the domains that have passed into the gel phase and remain in the liquid crystal state will be under different conditions. Relative to the equilibrium state, the molecules in the domain of the gel phase will be stretched, and in the liquid crystal state they will be compressed. An elastic stress will appear, which will lead to a violation of the bilayer structure.
Lipid pores and membrane permeability. In terms of permeability, lipid pores fundamentally differ from protein channels in their origin and exceptional dynamism. While protein channels have strictly defined sizes, which are maintained throughout the life of the cell, the sizes of lilid pores during the process of wicking vary widely. However, this variability; has a limit. If the pore radius is less than the critical one, then the pore in the process of flowing must pass through all intermediate radii and reach minimum size. The question of the possibility of complete wicking of lipid pores remains open. It is assumed that the complete tightening of the pore is prevented by powerful hydration forces, which manifest themselves when the walls of hydrophilic pores approach each other. Large pores, in contrast to protein ion channels, do not exhibit pronounced selectivity, which correlates with their relatively large initial sizes. It is clear, however, that lipid pores can reach arbitrarily small sizes during wicking, including those comparable to those of protein ion channels, which can lead to a redistribution of ion currents in the membrane, for example, upon excitation. It is further known that after the stress is turned off, the bilayer lipid membrane can return to a state of low conductivity, which implies that the pores reach a size insufficient for the passage of hydrated ions. Thus, hydrophilic lipid pores are universal in that they can be used by the cell for the transport of macromolecular substances, ions, and water molecules.
Studies of the permeability of lipid pores are currently developing in two directions: in the first one, the largest possible pores are studied, in the second, on the contrary, lipid pores of the minimum radius are studied. In the first case, we are talking about electro-transfection - a method of introducing DNA molecules into living cells or liposomes in order to transfer and intracellularly introduce foreign genetic material. It turned out that the external electric field high tension promotes the penetration of a giant DNA molecule inside the membrane particle. Maximum size The critical pore corresponds to the liquid-crystalline state of the lipid bilayer in the absence of an external electric field and is equal to 9 nm. The application of an external electric field with a strength of 100 kV/m reduces the critical pore radius to 1 nm in a time of 0.2 s. Since the membranes are preserved, the size of the lipid pores in them does not exceed this lower limit. The paradox is that the effective diameter of the statistical coil of DNA, which must fall into the particle, reaches 2000 nm. Therefore, the DNA molecule must penetrate the membrane in the form of an unraveled single strand. It is known that the end of the thread has a diameter of 2 nm and thus can only just enter the pore. However, free diffusion of the DNA strand in the pore is hardly possible in this case. Unfortunately, the mechanism of this phenomenon is not completely clear. It is assumed, in particular, that the DNA molecule is able to expand the pore and thus slip through the membrane. DNA penetration can be facilitated by additional forces of electrophoresis and electroosmosis, taking into account the total negative charge of the DNA molecule. It is possible that the pores with the ends of the DNA molecule fixed in them play the role of an anchor holding the molecule in a certain place near the surface of the vesicle membrane, and the transfer process itself is a type of pinocytosis. The study of this interesting phenomenon from the point of view of permeability continues,
The second direction in the study of membrane permeability involving lipid pores is related to the transmembrane transport of water molecules and ions. The well-known in biology phenomenon of high water permeability of cell membranes is fully reproduced on artificial lipid bilayers, which implies the participation of hydrophilic lipid pores in this process.
The main conclusion is that the stability of the lipid bilayer and the cell membrane devoid of a protein scaffold is determined by the lipid pores. These pores are formed at the sites of defects in the liquid crystal structure of the lipid bilayer. Lipid pores arise as a result of thermal fluctuations of the bilayer surface, and can also be generated during membrane stress that accompanies the phase transition of membrane lipids, during electrical breakdown and osmotic lysis. The fate of the membrane in these cases will depend probabilistically on whether the lipid pore exceeds some critical size or not. In the first case, the membrane will break, in the second case, its structure will be preserved. While maintaining the stability of the membranes, the pores are healed, running through all the intermediate values of the radii. The minimum radii of lipid pores can become comparable to the size of selective protein channels that normally regulate the ion permeability of cell membranes. In the last stages of wicking, lipid pores can turn into water pores accessible only to water molecules and ions.
Living cells, like the organism as a whole, are an open system with a constant exchange of matter and energy. In the process of this exchange, the penetration of substances into and out of the cell occurs.
With the formation of electrical potentials in cells, a violation of cell permeability leads to pathological changes, the therapeutic effect of the doctor with the appointment of the drug is associated with the penetration of these substances into the cell and the impact on its functional properties.
Tasks
- Study of the mechanism of penetration of substances through the membrane
- Determination of the distribution of substances between the intracellular and extracellular environment.
Permeability study methods
- Volume method. Based on the phenomenon of osmosis. Determination of the mass of cells before and after placing them in a hypertonic solution of the substance under study. Substance enters the cell and increases volume (due to water). Centrifugation method - determination of erythrocyte mass using it. With photometry, the rate of penetration of substances into the cell changes.
- indicator method. It boils down to the determination of intravital coloration. Qualitative method, because the intake of a substance is determined by the change in the color of the indicator, which is previously introduced into the cell. Used for acids and alkalis. The colorimetry method can give not only a qualitative, but also a quantitative assessment. The disadvantage is that small concentrations are poorly captured, and large concentrations are detrimental to the cell.
- Chemical method - investigates the qualitative and quantitative determination of substances in cells and the environment.
- Isotopic method for studying permeability. It allows you to study the flows of any substances entering both into the cell and out of it. The method allows working on living objects and using low concentrations of the studied substances. Allows you to study the penetration of not only foreign substances, but also substances - components of a given cell.
- Method for measuring electrical conductivity. Used to measure ions. By changing the low-frequency current allows you to judge the permeability.
Physical factors that cause passive penetration of substances through the membrane.
- Concentration (chemical) gradient
- electrochemical gradient
- Electrostatic gradient (for filtration processes)
- Osmotic Gradient
- Solubility gradient at the boundary of two immiscible phases, such as lipid and water
They provide passive movement of substances.
Active permeability comes with energy costs, the transfer is carried out against the concentration gradient.
The main type of passive transport will be diffusion - simple (through pores in the lipid bilayer, through a protein pore, or through pores in the lipid bilayer) and facilitated with (fixed or mobile carrier). Passive transport includes osmosis and filtration - the movement of a substance and a solvent.
Diffusion is the main way of transferring substances. Diffusion is a spontaneous process of penetration of substances from a larger area into an area with a lower concentration as a result of the thermal chaotic movement of atoms and molecules. Kinetic energy - mV2 / 2.
If the particle has a charge, an electrochemical gradient is also switched on.
Nernst-Planck equation
Jm = URT dC/dx - UCZF df/dx
U - particle mobility
C - concentration
R - gas constant
T- temperature
Z is the charge of the ion
F - Faraday number
Dx - membrane thickness
dC/dx - concentration gradient
dph/dx - electrochemical potential gradient
Fick's Law
Jm = -D dC/dx Jm = P(C1-C2)
If there is no charge.
P - membrane permeability coefficient
K - distribution coefficient
Substances in the process of diffusion pass through the pores of the membrane - water-soluble, polar compounds and electrolytes. Organic matter passes through dissolution in lipids. The dependence of the dissolution of substances in lipids was studied by Overton. He showed that if there are carboxyl, hydroxyl and amino groups, then this impairs the penetration through the membrane. The presence of methyl, ethyl and phenyl groups, on the contrary, facilitates the penetration of substances into the cell. They are not polar and this increases the dissolution of these substances in lipids.
The distribution coefficient shows the ratio of the solubility of substances in fats to the solubility of these substances in water. The higher this coefficient, the easier it is for substances to penetrate into the cell, regardless of the size of the molecule. If substances have the same partition coefficient, then smaller molecules will penetrate more easily than larger ones.
Water-soluble substances pass through the pores of the membranes. In order to pass through a pore, a substance must overcome certain forces that prevent it. The substance must be freed from the aqueous or solvate shell, push the surface molecular layer at the boundary of the cell and the washing solution, overcome the interaction of its polar groups and the polar groups of the membrane pores, overcome the energy barrier created on the surface of the cytoplasm by ions and colloids.
Ion permeability through the membrane.
It depends on the following factors.
- Crystal Radius Size
- The size of the hydration shell and its strength
- From the valency of the ion, which is determined by the magnitude of the charge
- From the phase transitions of the membrane from the liquid-crystal state to the gel and vice versa. The radius of a hydrated ion will be determined by the crystalline radius and the presence of one or more hydrated shells. The water shell of anions is 18% more compact than that of cations. Anions pass better through the membrane. When passing through a pore, an ion retains one hydration shell, while the rest are replaced by pore walls. This happens more easily if the energy of hydration is less.
The penetration into the cell will be affected by the charge, because. there is an interaction with the occasion. Monovalent ions are better than 2x and than 3x. Sodium, potassium are better, calcium, magnesium are better, iron is very bad.
membrane condition. Pores of liquid crystal and gel. Crystal (occupies a high density, due to the expansion of fat tails - 0.58 and 3.9). Gel fat tails are parallel and the area decreases to 0.48, but the thickness increases to 4.7. Trance configuration - stretched and deflected tails in gauche-trans-gauche configuration.
In the liquid-crystal state, there are microcavities in the membrane - a kink loop. these microcavities capture ions, water and they can move along the membrane and the membrane carries out the transfer.
The diffusion process can be facilitated by the presence of carriers. The feature of facilitated diffusion is the same as the concentration gradient, only faster. It has the property of a transport maximum - the increase in the permeability rate of a substance depends on free carriers, but when all carriers are occupied, the rate decreases. The increase in the rate of facilitated diffusion goes up to a certain moment. The competition of transported substances is possible, when different substances are attached to the carrier.
The process of filtration is the vision of a solution through a pore in a membrane under the action of a pressure gradient.
Obeys the Poiseuille equation
dV/dt = pi R4(p1-p2) / 8lή
dV/dt=(p1-p2)/ w W=8lή/pi R4
r4 - pore radius
l- pore length
ή - fluid viscosity
V - volume of filtered liquid
W - hydraulic resistance
In the capillaries of the glomeruli of the kidneys - rolls cannot pass through the filter, they remain in the plasma and create an osmotic pressure. The filtered liquid - creates the hydrostatic pressure preventing filtration.
Osmosis is of great importance in the body. Water, according to the laws of osmosis, from a solution with a lower concentration of substances into a solution with a higher concentration. Osmosis is the diffusion of water molecules. Idea by osmotic pressure gradient (pi)
Pi=iRCT i-isotonic coefficient of dissociation of molecules.
Osmolality. Osmol.
When determining the concentration of a solution in terms of particles, instead of harmms - osmol.
One osmol is 1 gram molecule of a solute.
A solution that contains 1/1000 osmol per 1 kg of water has an osmolality of 1 milliosmol (mosm) per 1 kg. Normal osmolality of extracellular and intracellular fluid is approximately 300 mosm per 1 kg
Osmolarity
Due to the difficulty of measuring water in a solution in kilograms, which is necessary to determine the osmolality, I use osmolarity instead - the concentration expressed as the number of osmoles per 1 liter of solution, and not per 1 kg.
Differences in osmolality and osmolality are less than 1%
Membrane permeability to water
- Osmotic Gradient
- hydrostatic gradient
- electrical gradient
- Oncotic pressure of proteins. Provides abnormal osmosis.
The unequal penetration rate of cations and anions creates a diffusion potential difference. This potential difference can affect the penetration of water. Abnormal osmosis can be positive or negative. With positive osmosis, water moves along the osmotic gradient, but with additional acceleration, and with negative anomalous osmosis, water moves against the osmotic gradient, but along the gradient of the electrical potential difference.
Theories of water transport
The van't Hoff theory is the penetration of water through the pores by the thermal movement of its molecules.
The penetration of water in the form of a vapor.
The membranes are highly permeable to gases, regardless of their nature. Gases do not have a charge. Gases can dissolve in lipids.
Permeability for acids and alkalis depends on the degree of their dissociation. Permeability of alkaloids too. Non-dissociable - pass well through the membrane, tk. dissolve in lipids, and dissociated ones cannot pass through the pores of the membranes due to their large size.
active transport- is associated with energy expenditure, and against the gradient.
Primary active and secondary active transport.
Primary active transport - pumping mechanisms for the transport of membrane ions.
The enzyme can be in 2 conformational states - E1/E2. May join alpha sub unit in state E1 three units. ATP breaks down to ADP and inorganic phosphate. The phosphate group is transferred to asparagine at position 376. During phosphorylation, the protein rotates and three ions from the inside are outside. The alpha sub unit after the turn acquires an affinity for potassium. And captures 2 potassium ions. Further dephosphorylation and a new conformational change transition to E2 and potassium 2 returns inside.
This transport maintains the normal distribution of sodium and potassium in the intracellular and extracellular fluids. Also + charge on outer surface membranes. With the removal of 3 sodium ions, water is removed from the cell, i.e. the water balance of the cell is maintained.
Secondary active transport is used to transport organic compounds necessary for the cell and this secondarily active transport is carried out with the help of carriers = 2 sodium + glucose (for example). Moves in the cell along the gradient of sodium into the cell. Here, energy is not consumed, but glucose from the cell must go into the blood - by simple diffusion, and sodium is removed from the cell by sodium-potassium ATPase. This is necessary to maintain the concentration gradient.
Active processes of substance transport are also associated with endocytosis - phagocyto - transfer of dense particles and pinocytosis - if liquids are transferred. This process may or may not be specific. Specific - if the membrane itself selects with the help of special membrane receptor proteins. The membrane forms a fold, which closes and turns into a vesicle, which creates the primary endosome, which includes a substance, a protein. Protein (clathrin) is removed from the primary endosome and the primary endosome passes into the secondary endosome, and it fuses with the lysosome.
Hormones that cannot pass through the membrane interact with receptors. And the other part of the fat-soluble hormones penetrates into the cell and interacts with cytosolic receptors.
A. Terminology. At present, different authors interpret the terms "permeability" and "conductivity" differently. Under the permeability of the cell membrane, we mean its ability to pass water and particles - charged (ions) and uncharged according to the laws of diffusion and filtration. The permeability of the cell membrane is determined by the following factors: 1) the presence of various ion channels in the membrane - controlled (with a gate mechanism) and uncontrolled (leak channels); 2) channel sizes and particle sizes; 3) the solubility of particles in the membrane (the cell membrane is permeable to lipids soluble in it and impermeable to peptides).
The term "conductivity" should only be used in relation to charged particles. Therefore, by conductivity we mean the ability of charged particles (ions) to pass through the cell membrane according to the electrochemical gradient (a combination of electrical and concentration gradients).
As is known, ions, like uncharged particles, pass through a membrane from a region of high concentration to a region of low concentration. With a large concentration gradient and good permeability of the membrane separating the corresponding solutions, the ion conductivity can be high, and one-way ion current is observed. When the concentration of ions on both sides of the membrane becomes equal, the ion conductivity will decrease, the one-way ion current will stop, although the permeability will remain the same - high. In addition, the conductivity of the ion at a constant membrane permeability also depends on the charge of the ion; charges of the same name repel, opposite charges attract, i.e. An important role in the conductivity of an ion is played by its electric charge. A situation is possible when, with good membrane permeability, the conductivity of ions through the membrane turns out to be low or zero, in the absence of a driving force (concentration and/or electrical gradients).
Thus, the conductivity of an ion depends on its electrochemical gradient and on the permeability of the membrane; the larger they are, the better the conductivity of the ion through the membrane. Movement of ions into and out of the cell according to concentration and electrical gradients cell at rest carried out primarily through unmanaged(without gate mechanism) channels (leak channels). Uncontrolled channels are always open, they practically do not change their capacity during electrical action on the cell membrane and its excitation. Unmanaged channels are divided into ion-selective channels (for example, potassium slow uncontrolled channels) and ion-nonselective channels. The latter pass various ions; K+, Ka+, C1".
B. The role of cell membrane permeability and various ions in the formation of PP(Fig. Z.2.).
| The vessel is separated by a semi-permeable membrane. Both halves of it are filled with Kr5O4 solutions of various concentrations (C| and SG), with C]< С2. Мембрана проницаема для иона К + и непроницаема для 8С>4 2 ~. Ions K + move according to the concentration gradient from the Savrasgvor C | solution. Since 8O4 ~ ions cannot pass into solution C], where their concentration is also lower, the membrane is polarized and an electric potential difference arises between its two surfaces, corresponding to the equilibrium potassium potential (Ek)- Ions R* and K + in a living cell at rest also move through the membrane according to the laws of diffusion, while K + leaves the cell in much greater quantities than Ka + enters the cell, since the permeability of the cell membrane for K * is approximately 25 times more permeability for Ka + . Organic anions, due to their large size, cannot leave the cell; therefore, inside the cell, at rest, there are more negative ions than positive ones. For this reason, the cell inside has a negative charge. Interestingly, at all points of the cell, the negative charge is almost the same. This is evidenced by the same RI value when the microelectrode is inserted at different depths into the cell, as was the case in the experiments of Hodgkin, Huxley, and Katz. Charge |
inside the cell is negative both absolutely (the cell hyaloplasm contains more anions than cations) and relative to the outer surface of the cell membrane.
Potassium is the main ion responsible for the formation of PP. This is evidenced by the results of the experiment with the perfusion of the internal contents of the giant squid axon with saline solutions. With a decrease in the concentration of K + ions in the perfusion solution, PP decreases, with an increase in their concentration, PP increases. In the resting state of the cell, a dynamic balance is established between the number of K + ions leaving the cell and entering the cell. Electrical and concentration gradients counteract each other: according to the concentration gradient, K + tends to leave the cell, the negative charge inside the cell and the positive charge of the outer surface of the cell membrane prevent this. When the concentration and electrical gradients are balanced, the number of K + ions leaving the cell is compared with the number of K + ions entering the cell. In this case, the so-called equilibrium potential.
Equilibrium potential for an ion can be calculated using the Nernst formula. The concentration of a positively charged ion outside the cell is written in the numerator in the Nernst formula, and the concentration of an ion inside the cell is written in the denominator. For negatively charged ions, the arrangement is opposite.
The contribution of Na + and Cl - to the creation of PP. The permeability of the cell membrane at rest for the N3+ ion is very low, much lower than for the K+ ion, however, it is present, therefore, the Ka* ions, according to the concentration and electrical gradients, strive and pass into the cell in a small amount. This leads to a decrease in PP, since the total number of positively charged ions on the outer surface of the cell membrane decreases, although slightly, and part of the negative ions inside the cell are neutralized by the positively charged Na + ions entering the cell. Ion entry Na+ inside cells reduces PP. The effect of SG on the PP value is opposite and depends on the permeability of the cell membrane for SG ions. The fact is that the SG ion, according to the concentration gradient, tends and passes into the cell. The electrical gradient prevents the entry of the SG ion into the cell, since the charge inside the cell is negative, as is the charge of SG. There comes an equilibrium between the forces of the concentration gradient, which promotes the entry of the SG ion into the cell, and the electrical gradient, which prevents the entry of the SG ion into the cell. Therefore, the intracellular concentration of SG ions is much less than the extracellular one. When the SG ion enters the cell, the number of negative charges outside the cell somewhat decreases, while inside the cell it increases: the SG ion is added to the large, protein-based anions located inside the cell. These anions, due to their large size, cannot pass through the channels of the cell membrane to the outside of the cell - into the interstitium. In this way, CI - ion, penetrating inside the cell, increases PP. Partially, as well as outside the cell, ions No + and C1" neutralize each other inside the cell. As a result, the joint entry of Na + and C1~ ions into the cell does not significantly affect the PP value.
C. Surface charges of the cell membrane itself and Ca 2+ ions play a certain role in the formation of PP. External and internal cell membrane surfaces carry their own electrical charges, predominantly negative. These are polar molecules of the cell membrane: glycolipids, phospholipids, glycoproteins. Fixed external negative charges, neutralizing the positive charges of the outer surface of the membrane, reduce the RI. Fixed internal negative charges of the cell membrane, on the contrary, adding up with anions inside the cell, increase the PP.
The role of Ca 2+ ions in the formation of PP lies in the fact that they interact with the external negative fixed charges of the cell membrane and neutralize them, which leads to an increase and stabilization of the PP.
In this way, PP- is the algebraic sum of not only all the charges of the ions outside and inside the cell, but also algebraic the sum of the negative external and internal surface charges of the membrane itself.
During measurements, the potential of the medium surrounding the cell is assumed to be zero. Relative to the zero potential of the external environment, the potential of the internal environment of the neuron, as noted, is about -60-80 mV. Cell damage leads to an increase in the permeability of cell membranes, as a result of which the difference in permeability for K + and N3 + ions decreases. At the same time, the PP decreases. Similar changes occur during tissue ischemia. In severely damaged cells, PP can decrease to the level of Donann equilibrium, when the concentration inside and outside the cell will be determined only by the selective permeability of the cell membrane in the resting state of the cell, which can lead to a disruption in the electrical activity of neurons. However, even normally, ions move according to the electrochemical gradient, but the PP is not disturbed.
INTRODUCTION
Membrane transport - the transport of substances through the cell membrane into or out of the cell, carried out using various mechanisms - simple diffusion, facilitated diffusion and active transport.
The most important property of a biological membrane is its ability to pass various substances into and out of the cell. This is of great importance for self-regulation and maintenance of a constant composition of the cell. This function of the cell membrane is performed due to selective permeability, i.e. the ability to pass some substances and not pass others. The easiest way to pass through the lipid bilayer is non-polar molecules with a small molecular weight (oxygen, nitrogen, benzene). Such small polar molecules as carbon dioxide, nitric oxide, water, and urea quickly penetrate through the lipid bilayer. Ethanol and glycerol, as well as steroids and thyroid hormones, pass through the lipid bilayer with a noticeable speed. For larger polar molecules (glucose, amino acids), as well as for ions, the lipid bilayer is practically impermeable, since its inner part is hydrophobic. So, for water, the permeability coefficient (cm/s) is about 10-2, for glycerol - 10-5, for glucose - 10-7, and for monovalent ions - less than 10-10.
The transport of large polar molecules and ions occurs due to channel proteins or carrier proteins. So, in cell membranes there are channels for sodium, potassium and chlorine ions, in the membranes of many cells there are water channels of aquaporins, as well as carrier proteins for glucose, various groups of amino acids and many ions. Active and passive transport.
Membranes form the structure of the cell and carry out its functions. Violation of the functions of the cell and intracellular membranes underlies the irreversible damage to cells and, as a consequence, the development serious illnesses cardiovascular, nervous, endocrine systems.
1. Basic facts about the structure of the cell membrane.
Cell membranes include plasmolemma, karyolemma, mitochondrial membranes, EPS, Golgi apparatus, lysosomes, peroxisomes. A common feature of all cell membranes is that they are thin (6-10 nm) layers of lipoprotein nature (lipids in combination with proteins). Main chemical components cell membranes are lipids (40%) and proteins (60%); in addition, carbohydrates (5-10%) were found in many membranes.
The plasma membrane surrounds each cell, determines its size and maintains the differences between the cell's contents and the external environment. The membrane serves as a highly selective filter and is responsible for the active transport of substances, that is, entry into the cell nutrients and the removal of harmful waste products. Finally, the membrane is responsible for the perception of external signals, allowing the cell to respond to external changes. All biological membranes are ensembles of lipid and protein molecules held together by non-covalent interactions.
The basis of any molecular membrane is made up of lipid molecules that form a bilayer. Lipids include a large group of organic substances that have poor solubility in water (hydrophobicity) and good solubility in organic solvents and fats (lipophilicity). The composition of lipids in different membranes is not the same. For example, the plasma membrane, unlike the membranes of the endoplasmic reticulum and mitochondria, is enriched with cholesterol. Characteristic representatives of lipids found in cell membranes are phospholipids (glycerophosphatides), sphingomyelins, and cholesterol from steroid lipids.
A feature of lipids is the division of their molecules into two functionally different parts: hydrophobic non-polar, non-charge-carrying ("tails"), consisting of fatty acids, and hydrophilic, charged polar "heads". This determines the ability of lipids to spontaneously form two-layer (bilipid) membrane structures with a thickness of 5-7 nm.
The first experiments confirming this were carried out in 1925.
Bilayer formation is a special property of lipid molecules and is realized even outside the cell. The most important properties of the bilayer: the ability to self-assembly - fluidity - asymmetry.
2. General ideas about permeability.
Characteristics of membranes, vessel walls and epithelial cells, reflecting the ability to conduct chemicals; distinguish between active (active transport of substances) and passive P. (phagocytosis
3. Transfer of molecules across the membrane.
Because the interior of the lipid layer is hydrophobic, it provides a virtually impenetrable barrier to most polar molecules. Due to the presence of this barrier, leakage of the contents of the cells is prevented, however, because of this, the cell was forced to create special mechanisms for the transport of water-soluble substances through the membrane. The transfer of small water-soluble molecules is carried out using special transport proteins. These are special transmembrane proteins, each of which is responsible for the transport of certain molecules or groups of related molecules.
In cells, there are also mechanisms for the transfer of macromolecules (proteins) and even large particles through the membrane. The process of absorption of macromolecules by the cell is called endocytosis. In general terms, the mechanism of its occurrence is as follows: local areas of the plasma membrane invaginate and close, forming an endocytic vesicle, then the absorbed particle usually enters the lysosomes and undergoes degradation.
3.1 Diffusion (Latin diffusio - distribution, spreading, scattering) - the process of transferring matter or energy from an area of high concentration to an area of low concentration (against the concentration gradient). The most famous example of diffusion is the mixing of gases or liquids (if you drop ink into water, the liquid will become uniformly colored after a while). Another example is related to a solid: if one end of the rod is heated or electrically charged, heat (or, respectively, electric current) spreads from the hot (charged) part to the cold (uncharged) part. In the case of a metal rod, thermal diffusion develops rapidly, and the current flows almost instantly. If the rod is made of synthetic material, thermal diffusion is slow, and diffusion of electrically charged particles is very slow. Diffusion of molecules proceeds in general even more slowly. For example, if a piece of sugar is lowered to the bottom of a glass of water and the water is not stirred, it will take several weeks before the solution becomes homogeneous. Even slower is the diffusion of one solid into another. For example, if copper is covered with gold, then gold will diffuse into copper, but under normal conditions (room temperature and atmospheric pressure), the gold-bearing layer will reach a thickness of several micrometers only after several thousand years.
All types of diffusion obey the same laws. The diffusion rate is proportional to the cross-sectional area of the sample, as well as the difference in concentrations, temperatures or charges (in the case of relatively small values of these parameters). Thus, heat will travel four times faster through a rod two centimeters in diameter than through a rod one centimeter in diameter. This heat will spread faster if the temperature difference per centimeter is 10°C instead of 5°C. The diffusion rate is also proportional to the parameter characterizing a specific material. In the case of thermal diffusion, this parameter is called thermal conductivity, in the case of a flow of electric charges - electrical conductivity. The amount of a substance that diffuses in a given time and the distance traveled by the diffusing substance are proportional to the square root of the diffusion time.
Diffusion is a process at the molecular level and is determined by the random nature of the movement of individual molecules. The diffusion rate is therefore proportional to the average velocity of the molecules. In the case of gases, the average speed of small molecules is greater, namely, it is inversely proportional to the square root of the mass of the molecule and increases with increasing temperature. Diffusion processes in solids at high temperatures often find practical applications. For example, certain types of cathode ray tubes (CRTs) use metallic thorium diffused through metallic tungsten at 2000°C.
3.2 Fick's equation
In most practical cases, the concentration C is used instead of the chemical potential. The direct replacement of µ by C becomes incorrect in the case of high concentrations, since the chemical potential is related to the concentration according to a logarithmic law. If we do not consider such cases, then the above formula can be replaced by the following.