Is it possible to apply three regol. Tri-regol: instructions for use, analogues and reviews, prices in Russian pharmacies
Triphasic oral contraceptive
Active ingredients
Ethinylestradiol (ethinylestradiol)
- levonorgestrel (levonorgestrel)
Release form, composition and packaging
Coated tablets, three types: round, biconvex, with a glossy surface; on a white break.
Tablets I, film-coated Pink colour(6 pieces in a blister).
Excipients: colloidal silicon dioxide - 0.275 mg, magnesium stearate - 0.55 mg, talc - 1.1 mg, corn starch - 19.995 mg, lactose monohydrate - 33 mg.
Shell composition: sucrose - 22.013 mg, talc - 6.935 mg, calcium carbonate - 2.898 mg, titanium dioxide - 1.814 mg, copovidone - 0.828 mg, macrogol 6000 - 0.207 mg, colloidal silicon dioxide - 0.123 mg, - 0.074 mg, carmellose sodium - 0.025 mg, iron oxide red - 0.083 mg.
Tablets II, white coated (5 pieces in a blister).
Excipients: colloidal silicon dioxide - 0.275 mg, magnesium stearate - 0.55 mg, talc - 1.1 mg, corn starch - 19.96 mg, lactose monohydrate - 33 mg.
Shell composition: sucrose - 22.013 mg, talc - 6.935 mg, calcium carbonate - 2.898 mg, titanium dioxide - 1.897 mg, copovidone - 0.828 mg, macrogol 6000 - 0.207 mg, colloidal silicon dioxide - 0.123 mg, povidone - 0.074 mg, carmellose sodium - 0.025 mg .
Tablets III, dark yellow coated (10 pieces in a blister).
Excipients: colloidal silicon dioxide - 0.275 mg, magnesium stearate - 0.55 mg, talc - 1.1 mg, corn starch - 19.92 mg, lactose monohydrate - 33 mg.
Shell composition: sucrose - 22.013 mg, talc - 6.935 mg, calcium carbonate - 2.898 mg, titanium dioxide - 1.317 mg, copovidone - 0.828 mg, macrogol 6000 - 0.207 mg, colloidal silicon dioxide - 0.123 mg, povidone - 0.074 mg, carmellose sodium - 0.025 mg , iron oxide yellow - 0.58 mg.
21 pcs. (tablets I, II, III) - blisters (1) - packs of cardboard.
21 pcs. (tablets I, II, III) - blisters (3) - packs of cardboard.
pharmachologic effect
Combined (three-phase) oral contraceptive estrogen-progestogen drug. Inhibits pituitary secretion of gonadotropic hormones. Sequential intake of tablets containing different amounts of progestogen (levonorgestrel) and estrogen (ethinyl estradiol) provides blood concentrations of these hormones close to their concentrations during a normal menstrual cycle and promotes the secretory transformation of the endometrium.
The contraceptive effect is associated with several mechanisms. Under the influence of levonorgestrel, a blockade of the release of releasing factors (LH and FSH) of the hypothalamus occurs, inhibition of the secretion of gonadotropic hormones by the pituitary gland, which leads to inhibition of the maturation and release of an egg ready for fertilization (ovulation). Ethinylestradiol maintains a high viscosity of cervical mucus (makes it difficult for spermatozoa to enter the uterine cavity). Along with the contraceptive effect, the menstrual cycle is normalized due to the replenishment of the level of endogenous hormones with the hormonal components of Tri-Regol tablets. In 7-day periods, when another break in taking the drug follows, uterine bleeding occurs.
Pharmacokinetics
Levonorgestrel
Suction
Levonorgestrel is rapidly absorbed (less than 4 hours). Does not undergo the effect of "first pass" through the liver.
Distribution and excretion
Most of the levonorgestrel in the blood binds to and with sex hormone-binding globulin. T 1/2 is 8-30 hours (average 16 hours). 60% of levonorgestrel is excreted by the kidneys, 40% - through the intestines.
Ethinylestradiol
absorption and metabolism
Ethinylestradiol is rapidly and almost completely absorbed from the gastrointestinal tract. Cmax in is achieved in the range of 1-1.5 hours. Ethinylestradiol undergoes a "first pass" effect through the liver. Metabolism is carried out in the liver and intestines.
breeding
When administered orally, ethinylestradiol is excreted from the blood plasma within 12 hours. Metabolites of ethinylestradiol: water-soluble derivatives of sulfate or glucuronide conjugation, enter the intestine with bile, where they are disintegrated by intestinal bacteria. 40% of ethinylestradiol is excreted by the kidneys and 60% through the intestines. T 1/2 is 26±6.8 hours.
Indications
- oral contraception.
Contraindications
- severe liver disease;
- liver tumors;
- congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes);
- cholelithiasis;
- cholecystitis;
- chronic colitis;
- the presence or indication of a history of severe cardiovascular (including decompensated heart defects) and cerebrovascular changes, thromboembolism and a predisposition to them;
- phlebitis of deep veins of the lower extremities;
- hormone-dependent malignant neoplasms of the genital organs and mammary glands (including suspicion of them);
- familial forms of hyperlipidemia;
- arterial hypertension with systolic/diastolic blood pressure 160/100 mm Hg. and higher;
- surgical interventions, surgical operations on the lower limbs;
- prolonged immobilization;
- extensive injuries;
- pancreatitis (including history), accompanied by severe hypertriglyceridemia and hyperlipidemia;
- jaundice due to medicines containing steroids;
- severe forms of diabetes;
- sickle cell anemia;
- chronic hemolytic anemia;
- vaginal bleeding of unknown etiology;
- migraine;
- cystic skid;
- otosclerosis with deterioration during the previous(their) pregnancy(s);
- idiopathic jaundice of pregnant women, severe pruritus of pregnant women;
- herpes of pregnant women in history;
- smoking over the age of 35;
- age over 40;
- lactase deficiency, lactose intolerance, glucose-galactose malabsorption (the dosage form of the drug contains lactose);
- pregnancy;
- lactation period;
- hypersensitivity to any component of the drug.
Carefully: compensated diabetes without vascular complications, arterial hypertension with systolic/diastolic blood pressure up to 160/100 mm Hg, varicose veins, multiple sclerosis, epilepsy, chorea minor, porphyria, tetany, bronchial asthma, adolescence(without regular ovulatory cycles), uterine fibroids, mastopathy, depression, tuberculosis.
Dosage
The drug should be taken orally, at the same time, if possible in the evening. Tablets are swallowed whole, without chewing and washing down with a small amount of liquid.
For the purpose of contraception in the first cycle, Tri-Regol is prescribed 1 tablet daily for 21 days, starting from the 1st day of the menstrual cycle, then a 7-day break is taken, during which menstrual bleeding occurs. The next package containing 21 film-coated tablets should be started on the 8th day after a 7-day break.
The drug is taken as long as there is a need for contraception.
When switching from another oral contraceptive to taking Tri-Regol a similar scheme applies.
Reception should begin no earlier than the 1st day of the menstrual cycle.
During lactation, the use of the drug is contraindicated.
If a woman has not taken Tri-Regol in set time , you should take the missed pill within the next 12 hours. If 36 hours have passed after taking the pill, contraception cannot be considered reliable. However, to avoid intermenstrual spotting it is necessary to continue taking the drug from the already started package, minus the missed tablet(s). At this time, it is recommended to additionally use another, non-hormonal method of contraception (for example, barrier).
Side effects
Side effects observed with the use of the drug are classified into categories depending on the frequency of their occurrence: very often (≥1/10), often (≥1/100,<1/10), иногда (≥1/1000, <1/100), редко (≥1/10 000, <1/1000), очень редко (<1/10 000), включая отдельные случаи.
From the reproductive system: possibly - engorgement of the mammary glands, decreased libido, intermenstrual bleeding; rarely - increased vaginal discharge, vaginal candidiasis.
From the digestive system: possibly - nausea, vomiting; rarely - jaundice, hepatitis, liver adenoma, gallbladder disease (eg, cholelithiasis, cholecystitis), diarrhea.
From the nervous system: possibly - headache, depressed mood; with prolonged use, very rarely - an increase in the frequency of epileptic seizures.
From the sense organs: in some cases - swelling of the eyelids, conjunctivitis, blurred vision, discomfort when wearing contact lenses (these phenomena are temporary and disappear after cancellation without prescribing any therapy); with prolonged use is very rare - hearing loss.
From the side of metabolism: possibly - an increase in body weight; rarely - an increase in the concentration of triglycerides in the blood, a decrease in glucose tolerance.
From the skin and subcutaneous tissues: possibly - chloasma; rarely - skin rash, hair loss; very rarely with prolonged use - generalized itching.
Others: rarely - increased fatigue, increased blood pressure, thrombosis and venous thromboembolism; with prolonged use, very rarely - cramps of the calf muscles, coarsening of the voice.
Overdose
Symptoms: nausea, uterine bleeding.
Treatment: when the first signs of an overdose appear in the first 2-3 hours, gastric lavage and symptomatic treatment are recommended. There is no antidote.
drug interaction
Tri-Regol should be used with caution concomitantly with the following medicines.
When used simultaneously with Tri-Regol, ampicillin, rifampicin, chloramphenicol, neomycin, sulfonamides, tetracyclines, dihydroergotamine, tranquilizers, phenylbutazone can weaken the contraceptive effect. With combinations, it is recommended to additionally use a different, non-hormonal method of contraception.
With the simultaneous use of the drug Tri-Regol with coumarin or indandione derivatives, an extraordinary determination of the prothrombin index and a change in the dose of the anticoagulant may be required.
With the simultaneous use of the drug Tri-Regol and tricyclic antidepressants, maprotiline, beta-blockers, an increase in bioavailability and, therefore, an increase in toxicity is possible.
With the simultaneous use of the drug Tri-Regol and oral hypoglycemic drugs, insulin, it may be necessary to change their doses.
With the simultaneous use of the drug Tri-Regol and bromocriptine, the effectiveness of the latter decreases.
With the simultaneous use of the drug Tri-Regol and drugs with a possible hepatotoxic effect, primarily dantrolene, the risk of increased hepatotoxicity increases, especially in women over 35 years of age.
special instructions
Before starting the use of the drug, it is necessary to exclude pregnancy, conduct a general medical and gynecological examination (examination of the mammary glands, cytological analysis of the smear).
While taking the drug requires a regular gynecological examination every 6 months.
The use of oral contraceptives is allowed no earlier than 6 months after the viral hepatitis and subject to the normalization of hepatic functions.
If there is a sharp pain in the upper abdomen, hepatomegaly, or signs of intra-abdominal hemorrhage, a liver tumor may be suspected. In this case, the drug should be discontinued.
With the appearance of acyclic spotting, it is possible to continue taking the drug Tri-Regol after the exclusion of organic pathology by the attending physician.
If violations of liver function are detected during the use of the drug, the question of the advisability of continuing to take Tri-Regol should be decided.
In case of vomiting or diarrhea, the drug should be continued, and it is recommended to additionally use another, non-hormonal method of contraception.
At least 3 months before the planned pregnancy, the drug should be stopped.
Under the influence of oral contraceptives (due to the estrogen component), some laboratory parameters may change (functional parameters of the liver, kidneys, adrenal glands, thyroid gland, blood coagulation and fibrinolytic factors, levels of lipoproteins and transport proteins).
The drug should be stopped immediately in the following cases:
- with a first-time or increased migraine-like or unusually severe headache, with an acute deterioration in visual acuity, with suspected thrombosis or heart attack;
- with a sharp increase in blood pressure, the appearance of jaundice or hepatitis without jaundice, the occurrence of generalized itching or an increase in epileptic seizures;
- at the onset of pregnancy;
- 6 weeks before the planned operation, with prolonged immobilization (for example, after injuries).
Influence on the ability to drive vehicles and mechanisms
Taking the drug does not affect the ability to drive a car and work with other mechanisms.
Pregnancy and lactation
Tri-Regol is contraindicated during pregnancy and lactation.
Application in childhood
FROM caution: adolescence (without regular ovulatory cycles).
For impaired liver function
Contraindicated in severe liver disease, liver tumors.
Use in the elderly
Contraindicated in people over 40 years of age.
Terms of dispensing from pharmacies
The drug is dispensed by prescription.
Terms and conditions of storage
The drug should be stored out of the reach of children at a temperature not exceeding 25 ° C. Shelf life - 2 years.
Thanks
The site provides reference information for informational purposes only. Diagnosis and treatment of diseases should be carried out under the supervision of a specialist. All drugs have contraindications. Expert advice is required!
Tradename
Tri-Regol(Tri-regol).Pharmacological group
Hormonal oral contraceptive.Release form and composition
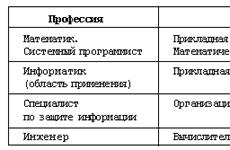
The drug Tri-regol is available in three dosage forms: dragees, tablets and coated tablets.One blister contains 21 tablets of three colors: pink - 6 pieces, white - 5 pieces, dark yellow - 10 pieces; packed in a cardboard box. 1 blister of Tri-regol 21 and 7 contains 7 reddish-brown placebo tablets (substance without medicinal properties).
1 pink tablet includes ethinylestradiol 30 mcg and levonorgestrel 50 mcg. Excipients included in the tablet: lactose monohydrate, colloidal silicon dioxide, talc, magnesium stearate and corn starch. Components of the shell: sucrose, calcium carbonate, talc, copovidone, titanium dioxide (E171), colloidal silicon dioxide, macrogol 6000, povidone, iron oxide red (E172), carmellose sodium. Excipients and shell components are the same for tablets of all colors.
1 white tablet includes levonorgestrel 75 mcg and ethinylestradiol 40 mcg.
1 dark yellow tablet includes levonorgestrel 125 mcg and ethinylestradiol 30 mcg.
1 placebo tablet contains iron fumarate - 76.05 mg. Potato starch is added to auxiliary substances.
Description of the drug Tri-regol
The drug Tri-regol is a round tablet coated with three colors (pink, white and dark yellow). The outer surface of the tablet is glossy, inside the substance is white.Placebo tablets are produced with a glossy surface of a round shape, on a brown break.
Pharmacological action of the drug Triregol
PharmacodynamicsTri-regol belongs to the group of contraceptive combined (three-phase) estrogen-gestagenic drugs. The gestagenic component is represented by levonorgestrel, the estrogenic component is ethinyl estradiol.
Levonorgestrel, by reducing the production of gonadotropic hormones, leads to a decrease in the rate of maturation of the egg, and prevents ovulation. Ethinylestradiol reduces the possibility of passage of spermatozoa into the uterine cavity, increasing the viscosity of cervical mucus.
One of the effects of the drug is the normalization of the menstrual cycle, since Tri-regol supplements the level of endogenous hormones due to its components.
Pharmacokinetics
Completely absorbed in the intestine when taken orally. It is processed in the liver and intestines, excreted by the kidneys (60% levonorgestrel and 40% ethinylestradiol) and through the intestines (40% levonorgestrel and 60% ethinylestradiol).
Indications for use
 In gynecological practice, the drug Tri-regol is prescribed for:
In gynecological practice, the drug Tri-regol is prescribed for:
- pregnancy prevention (contraception);
- treatment of dysfunctional metrorrhagia;
- treatment of non-organic dysmenorrhea;
- treatment of premenstrual syndrome;
- treatment of menstrual disorders.
Contraindications for use
The appointment of the drug Tri-regol is contraindicated in the presence of the diseases indicated below:- pregnancy and lactation;
- the presence of hypersensitivity to any component of the drug in the form of allergic reactions;
- a history of severe itching and severe idiopathic jaundice in pregnancy;
- liver disease (liver tumors, hepatitis, Gilbert, Rotor and Dubin-Johnson syndromes in history, liver failure);
- gallbladder diseases: cholelithiasis, cholecystitis;
- age over 40, smoking at the age of 35 and over;
- severe cardiovascular (severe arterial hypertension, myocarditis, decompensated heart defects, decompensated chronic heart failure) and cerebrovascular (hemorrhagic and ischemic stroke) changes in history, thromboembolism and thrombosis, as well as predisposition to them;
- vaginal bleeding of unknown etiology;
- severe diabetes mellitus;
- sickle cell and hemolytic anemia;
- disorders of fat metabolism;
- malignant tumors, especially endometrial or breast cancer;
- cystic skid;
- chronic colitis;
- otosclerosis with hearing impairment;
- inflammation of the walls of the deep veins of the lower extremities (phlebitis);
- surgical operations (especially on the legs);
- extensive injuries and prolonged immobilization;
- lactase deficiency (insufficiency of the enzyme necessary for the digestion of milk lactose).
- diabetes mellitus in the compensation stage without vascular complications;
- chronic venous insufficiency of the lower extremities;
- arterial hypertension with blood pressure up to 160/100 mm Hg;
- chorea;
- porphyria;
- lack of regular ovulatory cycles in adolescence.
Dosing regimen of the drug Tri-regol
 Triregol is taken orally. The tablets must be swallowed whole with a small amount of water. It is better to take tablets in the evening, the interval between doses should not exceed 36 hours.
Triregol is taken orally. The tablets must be swallowed whole with a small amount of water. It is better to take tablets in the evening, the interval between doses should not exceed 36 hours. For the purpose of contraception, Triregol must be taken on the first day of the menstrual cycle. Duration of admission is 21 days followed by a break of 7 days. In the interval between doses, moderate bleeding is noted, as with menstruation. Tablets are taken in strict order, they are numbered according to the days of the menstrual cycle. The drug should be continued on the 8th day. Reception of the drug Tri-regol 21+7 is carried out continuously.
If you miss a tablet, you must take it within 12 hours, no later. With a break in taking Tri-regol contraceptive pills for 36 hours or more, the drug is continued according to the scheme, excluding the missed pill (it is not taken). In this case, pregnancy is possible, therefore, along with taking Tri-regol, other methods of contraception (preferably barrier ones) should be used.
The remedy is taken all the time as long as prevention of pregnancy is necessary.
With a therapeutic purpose, the doctor selects the dose individually in each specific case.
Side effects of the drug Triregol
When using Tri-regol, nausea, vomiting, headache, mastalgia (breast engorgement and tenderness), mood lability and fatigue, changes in body weight, intermenstrual bleeding, conjunctivitis and temporary visual impairment are possible as side effects.Rare side effects include an increase in the concentration of glucose and triglycerides in the blood serum, an increase in blood pressure, changes in the liver (jaundice, hepatitis, liver adenoma), gallbladder disease (cholecystitis, cholelithiasis), thrombosis and venous thromboembolism, skin rash, hair loss , vaginal candidiasis, diarrhea.
Very rarely, mainly with prolonged use of the drug, the occurrence of cramps in the calf muscles, generalized itching, coarsening of the voice, an increase in the frequency of epileptic seizures, and hearing loss are noted.
Symptoms of an overdose with Tri-regol
As a result of an overdose of the drug, nausea and uterine bleeding are noted. In this case, it is necessary to consult a doctor (for gastric lavage and symptomatic therapy.Interaction of the drug Triregol with other drugs
means
Ampicillin, chloramphenicol, rifampicin, polymyxin B, neomycin, sulfonamides, dihydroergotamine, tetracyclines, tranquilizers, phenylbutazones can reduce the contraceptive effect when taken simultaneously with Tri-Regol, disrupting the balance of microflora. When taken simultaneously, Tri-Regol enhances the side effects of hepatotoxic drugs (primarily dantrolene), especially in women over 35 years of age.
When taking the drug Triregol, it may be necessary to correct the intake of indirect anticoagulants and hypoglycemic drugs, including insulin.
In the case of diarrhea or vomiting, the absorption of the drug in the intestine is reduced, which leads to a decrease in the contraceptive effect. The drug should be continued, but it is necessary to additionally use a non-hormonal method of contraception.
If moderate bleeding occurs, it is not necessary to stop the course of taking the drug Triregol.
It is necessary to immediately stop the course:
- with a first-time, or becoming more intense, migraine-like headache;
- at the onset of pregnancy;
- with the appearance of stabbing pains when coughing or breathing, feelings of tightness and pain in the chest;
- with acute deterioration in visual acuity;
- with an increase in epileptic seizures;
- with suspicion of thrombosis;
- if a liver tumor is suspected (aching pain in the right hypochondrium, jaundice, liver enlargement);
- with prolonged immobilization after injuries;
- 6 weeks before planned surgery.
The use of the drug Triregol is contraindicated during lactation (excreted in small amounts with breast milk) and during pregnancy. Three months before the onset of a planned pregnancy, you should stop taking the drug.
Driving vehicles and working with mechanisms
The drug Tri-regol does not affect the speed of physical and mental reactions.
Analogues of the drug Tri-regol

- Triquilar is a therapeutic three-phase contraceptive drug. The active substances and the principle of action are similar to Tri-regol. Manufacturer: Schering, Germany.
- Triziston refers to therapeutic combined estrogen-gestagen preparations. The action and therapeutic components are identical to Tri-regol, the dosage of active substances is different. Women with regular heavy loads on the vocal cords (professional lecturers, announcers) should not take the drug. Manufacturer: Schering, Germany.
- Ovidon is a monophasic combined contraceptive drug. It is indicated for women of the estrogen phenotype (feminine appearance), since the content of levonorgestrel is increased in the drug. Producer: Gedeon Richter, Hungary.
Terms and conditions of storage
Shelf life - 5 years. After the expiration date, the drug should not be used.Tri-regol refers to drugs from list B, which are stored with caution, out of the reach of children and at a temperature of 15 to 30 ° C.
Conditions for dispensing in pharmacies of the drug Tri-regol
The drug Tri-regol in the pharmacy network can only be purchased with a doctor's prescription.The price of the drug Tri-regol
The average price for Tri-regol in the Moscow pharmacy chain is:Tri-regol birth control pills - 163.78 rubles;
Tri-regol contraceptive pills, coated 21+7 - 460.53 rubles.
Compound
Active ingredients: ethinylestradiol, levonorgestrel;
1 pink tablet contains ethinylestradiol 0.03 mg, levonorgestrel 0.05 mg
1 white tablet contains ethinylestradiol 0.04 mg, levonorgestrel 0.075 mg
1 dark yellow tablet contains ethinyl estradiol 0.03 mg, levonorgestrel 0.125 mg
Excipients: colloidal silicon dioxide, magnesium stearate, talc, corn starch, lactose, carmellose sodium, povidone, polyethylene glycol (macrogol 6000), iron oxide red (E172), iron oxide yellow (E172), copolyvidone, titanium dioxide (E 171) , calcium carbonate, sucrose.
Dosage form
Coated tablets.
Basic physical and chemical properties: pink round biconvex film-coated tablets with a glossy surface.
White, round, biconvex film-coated tablets with a glossy surface.
Dark yellow, round, biconvex film-coated tablets with a glossy surface.
Pharmacological group
Hormonal contraceptives for systemic use.
Pharmacological properties
Pharmacological.
Combined oral contraceptives block the action of gonadotropins. The primary action of these drugs is aimed at inhibiting ovulation. The drug causes a change in cervical mucus, which makes it difficult for sperm to pass into the uterine cavity and affects the endometrium, thereby reducing the possibility of implantation of a fertilized egg. All this contributes to the prevention of pregnancy.
Pharmacokinetics.
Levonorgestrel.
Absorption: when taken, levonorgestrel is rapidly and completely absorbed from the gastrointestinal tract. Bioavailability - almost 100% due to the lack of primary metabolism.
Distribution Most of levonorgestrel binds to plasma proteins, mainly to albumin and sex hormone-binding globulin.
Metabolism: Basically, it consists in the elimination of the Δ 4-3-oxo group and hydroxylation at positions 2 α, 1b and 16b, after which conjugation occurs. Most of the metabolites that circulate in the blood are 3α,5b-tetrahydro-levonorgestrel sulfates. Excretion of the drug occurs mainly in the form of glucuronides. Some primary levonorgestrel also circulates as 17b-sulfate. Metabolic clearance is marked by individual variability, which may partly explain the significant differences in levonorgestrel concentrations that are observed in patients.
Conclusion: The half-life of levonorgestrel shows individual variability and is approximately 36 hours at steady state. Levonorgestrel is excreted in the urine
(40-68%) and feces (16-48%) in the form of metabolites (sulfate and conjugates with glucuronic acid).
Ethinylestradiol.
Absorption: ethinylestradiol is absorbed rapidly and almost completely, the maximum concentration in blood serum is reached after 1.5 hours. After presystemic conjugation and metabolism, the absolute bioavailability is 60%. The area under the curve and Cmax may increase slightly over time.
Distribution Ethinylestradiol is 98% bound to plasma proteins, mainly to albumin.
Metabolism Ethinylestradiol is cleaved by presystemic conjugation. Passes through the intestinal wall (first phase of metabolism) and enters the liver, where conjugation occurs (second phase of metabolism). The most important metabolites of the first phase of metabolism are 2-OH-ethinylestradiol and 2-methoxy-ethinylestradiol. Both ethinylestradiol and the metabolites of the first phase are excreted as conjugates (sulfates and glucuronides) into the bile and enter the hepato-intestinal circulation.
Conclusion: ethinylestradiol is excreted from blood plasma with an elimination half-life, which averages 29 hours (26-33 hours); plasma clearance varies in the range of 10-30 l/hour. Withdrawal of conjugates of ethinylestradiol and its metabolites with urine and feces in a ratio of 1: 1.
Indications
Oral contraception.
Contraindications
Combined oral contraceptives (COCs) are not recommended for use in the presence of diseases and pathological conditions listed below. With the development of such diseases with the use of COCs, the drug should be stopped immediately:
- pregnancy or suspected pregnancy, breastfeeding period
- hypersensitivity to the components of the drug
- the presence or references in history to arterial or venous thromboembolic diseases (for example, deep vein thrombophlebitis, pulmonary embolism) in combination with or without risk factors (see section "Peculiarities of use");
- the presence of a risk of arterial or venous thromboembolism (blood clotting disorders, heart disease, atrial fibrillation, cerebrovascular disorders, myocardial infarction);
- a history of prodromal symptoms of thrombosis (transient attack of ischemia, angina pectoris);
- the presence or indication of a history of cerebrovascular accident
- severe degree or the presence of multiple risk factors for venous or arterial thrombosis can currently be considered a contraindication (see section "Peculiarities of use");
- cardiovascular diseases (heart disease, heart rhythm disturbances, heart valve pathology)
- severe course of hypertension;
- diabetes mellitus with vascular disorders;
- ophthalmic disorders of vascular origin;
- severe liver disease present or in history until liver function tests return to normal;
- presence or history of liver tumors (benign or malignant)
- migraine in history with focal neurological symptoms;
- diagnosed or under suspicion of malignant tumors that are caused by sex steroids (for example, genital or mammary glands);
- vaginal bleeding of unknown etiology.
Interaction with other medicinal products and other forms of interaction
Interactions between COCs and other medicinal products may result in impaired contraceptive effectiveness and/or breakthrough bleeding and/or ineffectiveness of this contraceptive method.
Women who are taking any of these drugs are advised to temporarily use a barrier or other method of contraception in addition to COCs. When taking drugs that induce liver enzymes, the barrier method is required for use during the entire course of treatment with such drugs and for 28 days after its completion.
For women who are taking antibiotics (with the exception of rifampicin and griseofulvin), it is recommended to use the barrier method during the period of antibiotic treatment and for 7 days after its completion.
If concomitant drug therapy is continued after the end of the tablets from a pack of COCs, the next pack of COCs should be started without the usual interruption.
Hepatic metabolism: Interactions may occur with drugs that induce microsomal enzymes, increasing the clearance of sex hormones (eg, phenytoin, barbiturates, primidone, carbamazepine, rifampicin, and possibly also oxcarbazepine, topiramate, felbamate, griseofulvin, and drugs containing St. John's wort (Hypericum perforatum).
In addition, there have been reports that HIV protease inhibitors (eg, ritonavir) and non-nucleoside reverse transcriptase inhibitors (eg, nevirapine) and combinations thereof may increase hepatic metabolism.
Hepatic recirculation: There is information that hepatic estrogen recirculation may increase when certain antibiotics (eg, penicillin, tetracycline) are prescribed as concomitant medications, which may lead to a decrease in serum ethinyl estradiol concentrations.
Troleandomycin may increase the risk of intrahepatic cholestasis when administered concomitantly with COCs.
The mechanism of their action, based on the ability of these substances to increase the activity of liver enzymes. The maximum induction of enzymes, as a rule, is observed no earlier than 2-3 weeks after the start of the use of these drugs, but may persist for at least 4 weeks after their withdrawal. Cases of contraceptive failure have also been reported with concomitant use of antibiotics such as ampicillin and tetracycline, but the mechanism of action remains unknown.
In case of short-term use of any of these drugs that cause an increase in liver enzymes, it is recommended to use additional barrier methods of contraception from the moment the use of these drugs is started, during the entire period of treatment and within 4 weeks after their withdrawal. Women who receive these antibiotics in a short course should temporarily use barrier methods of contraception simultaneously with contraceptive pills, that is, during the period of use of the concomitant medicinal product and within 7 days after its withdrawal. If the next package of Tri-Regol tablets ends earlier than the period of time that requires the use of additional contraceptives, you should start the tablets from the next package without interrupting the use of the drug. In this case, "withdrawal bleeding" should not be expected until the pills from the second package run out. If the patient does not experience "withdrawal bleeding" after finishing taking the pills from the second pack, she should consult a doctor to rule out pregnancy. In the case of long-term use of these drugs, patients are advised to use other contraceptives.
St. John's wort (Hypericum perforatum) herbal products are not recommended to be administered simultaneously with these drugs, as this leads to a potential decrease in the contraceptive effect of Tri-Regol tablets. There have been reports of breakthrough bleeding and unintended pregnancy. The decrease in the contraceptive effect persists for less than 2 weeks after stopping treatment with St. John's wort.
There have been reports of elevated plasma concentrations of cyclosporine with concomitant use of COCs. The CPC was found to be able to induce the metabolism of lamotrigine resulting in sub-therapeutic plasma levels of lamotrigine.
Laboratory research. The use of steroidal contraceptives may affect the results of certain laboratory tests, including biochemical parameters of liver, thyroid, adrenal and kidney function at the level of plasma proteins, for example, GCS-binding globulin and lipid / lipoprotein fraction; indicators of carbohydrate metabolism and indicators of blood coagulation and fibrinolysis. Changes usually do not exceed the laboratory limits of the norm.
Application features
Medical examination / consultation.
Before starting the use of the drug or re-prescribing the drug, it is necessary to collect a detailed family history and conduct a general medical and gynecological examination (primarily measuring blood pressure, determining blood and urine sugar levels, examining liver function, examining the mammary glands, cytological smear analysis) to rule out dressings associated with the risk of disease and pregnancy. The frequency and nature of examinations should be based on established clinical guidelines on a case-by-case basis.
A woman must be warned that the drug does not protect her from sexually transmitted infections, in particular from AIDS.
Special Warnings.
Smoking increases the risk of developing serious adverse reactions from the cardiovascular system against the background of the use of COCs. This risk increases with age, depends on the number of cigarettes smoked, and is especially high in women over the age of 35. All women who use COCs should be strongly advised to stop smoking. Women over the age of 35 who smoke should consider other methods of contraception.
In the presence of any of the diseases/risk factors listed below, the beneficial effects of COCs and the possible risks of their use in an individual woman should be evaluated and the respective benefits and risks should be discussed with her before she decides to use such drugs. In case of the first manifestation, worsening or exacerbation of any of these diseases or risk factors, a doctor should be consulted. Then the doctor must decide to stop taking the COC.
Circulatory disorders.
Epidemiological studies have shown that the incidence of venous thromboembolism (VTE) in women using low-estrogen oral contraceptives (<50 мкг этинилэстрадиола) составляет 20-40 случаев из 100 000 женщин в год, но этот риск варьируется в зависимости от количества прогестагена. Это равно 5-10 случаев с 100000 женщин в год для женщин, которые не применяют КПК. Применение любого комбинированного противозачаточного препарата увеличивает риск венозных тромбоэмболических заболеваний (ВТЗ) по сравнению с данными показателями у женщин, которые не используют КПК.
The risk of these diseases reaches a maximum in the first year of drug use. This increased risk is less than the risk of venous thromboembolic disease detected during pregnancy is 60 cases per 100,000 pregnancies (1-2% of these cases are fatal).
In general, the probability of occurrence of thromboembolic diseases with the use of oral contraceptives containing levonorgestrel and 30 micrograms of ethinyl estradiol is 20 cases per 100,000 women per year.
Epidemiological studies have also associated the use of combined COCs with an increased risk of myocardial infarction, transient ischemic attack, and stroke.
Very rarely, there have been reports of thrombosis of other blood vessels, such as hepatic, mesenteric, renal, retinal veins, and arteries, in women taking birth control pills. The connection between the development of these phenomena and the use of hormonal contraceptives has not been proven.
The risk of thromboembolism (arterial and / or venous) and cerebral circulation increases:
- with age;
- when smoking (excessive smoking and age, especially over 35, are additional risk factors);
- with a burdened family history (for example, diseases of the father or brother, sister at a young age). If there is a congenital tendency to thromboembolic diseases, it is necessary to consult a specialist before using the drug.
- with obesity (body mass index above 30 kg / m 2);
- in violation of fat metabolism (dyslipoproteinemia)
- with arterial hypertension;
- with migraine
- in valvular heart disease
- with atrial fibrillation (atrial fibrillation)
- with prolonged immobilization, severe operations, operations on the lower extremities, severe injuries. Due to the fact that the risk of thromboembolic diseases increases in the postoperative period, it is proposed to stop taking the drug 4 weeks before surgery and start taking it 2 weeks after the patient's remobilization;
- there is no consensus on the possible role of varicose veins and superficial thrombophlebitis in the development or progression of venous thrombosis.
Symptoms of venous or arterial thrombotic / thromboembolic disease or symptoms of a cerebrovascular accident may include:
- unusual unilateral pain and/or swelling of the legs;
- sudden sharp pain in the chest, regardless of whether it spreads to the left arm;
- sudden respiratory failure
- sudden cough for no apparent reason;
- any unusual, acute or prolonged headache
- sudden partial or complete loss of vision;
- diplopia;
- slurred speech or aphasia;
- vertigo;
- collapse with or without focal epileptic seizure;
- weakness or very severe numbness that suddenly affects one side or one part of the body
- movement disorders;
- "Acute belly".
The use of COCs has generally been associated with an increased risk of acute myocardial infarction or apoplexy. The mechanisms of the effect of Tri-Regol on the risk of developing acute myocardial infarction have not been studied.
In the postpartum period, an increased risk of venous thromboembolism should be taken into account (see section "Use during pregnancy or lactation").
Other diseases associated with adverse circulatory reactions include diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis), and sickle cell anemia.
In the event of an increase in the frequency or severity of migraine when using oral contraceptives (which may be a precursor or a stroke event), it may be a reason for immediate discontinuation of the drug.
Biochemical factors that may indicate a congenital or acquired predisposition to venous or arterial thrombosis include resistance to activated protein C, factor V Leiden mutation, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, the presence of antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant) and dyslipoproteinemia.
Some studies have documented an increase in the incidence of cervical cancer among women who have taken combined oral contraceptives for a long time, but the results are mixed. Sexual behavior and other factors, such as the human papillomavirus, are involved in the formation of cervical cancer, so the relationship between cervical cancer and the use of combined oral contraceptives is ambiguous.
An analysis of epidemiological studies has shown that women who use COCs have a slightly increased relative risk of developing breast cancer. This increased risk gradually decreases over 10 years after the cessation of COC use. Since breast cancer is rare in women younger than 40 years of age, the increase in the number of diagnosed cases of breast cancer in women who use COCs now or in the past is small compared to the risk of developing breast cancer over the entire period of life.
Evidence of a causal relationship was not presented in these studies. The increased risk may be due to early detection of breast cancer in women using COCs, the biological effects of COCs, or a combination of both.
Women who use oral contraceptives are diagnosed with breast cancer at a slightly earlier stage than women who do not use COCs.
With prolonged use of sex hormones, benign, very rarely, malignant liver tumors have occasionally been observed, which in some cases can lead to life-threatening bleeding in the abdominal cavity. With the appearance of severe acute pain in the upper abdomen, enlargement of the liver, or signs of intraperitoneal bleeding, a liver tumor may be suspected. This must be taken into account when making a differentiated diagnosis.
Other diseases.
Women with or a family history of hypertriglyceridemia are at an increased risk of pancreatitis when taking COCs. Women with hyperlipidemia should be under close medical supervision if they decide to use COCs.
A slight increase in blood pressure has been reported in many women who have taken COCs, but clinically significant increases have been rare. Only in these rare cases has immediate discontinuation of COCs been justified. If COC use in pre-existing hypertension results in persistently elevated blood pressure levels or a significant increase in blood pressure that is not adequate for antihypertensive treatment, COC use should be discontinued. In some cases, COC use can be restored if normal blood pressure values can be achieved with antihypertensive therapy.
Although the evidence of association with the use of COCs is inconclusive, there have been reports of the development or exacerbation of such diseases during pregnancy and when taking COCs: jaundice and / or itching associated with cholestasis; the formation of stones in the gallbladder; porphyria; systemic lupus erythematosus, hemolytic uremic syndrome of Sydenham's chorea; herpes pregnancy hearing loss associated with otosclerosis.
In women with hereditary angioedema, estrogen intake may induce or exacerbate the symptoms of angioedema.
Acute or chronic liver dysfunction may require discontinuation of COCs until liver function tests return to normal. Recurrence of cholestatic jaundice and/or pruritus that occurred during pregnancy or previous use of sex steroid hormones requires discontinuation of COCs.
Although COCs may affect peripheral insulin resistance and glucose tolerance, there is no evidence for the need to change the dosing regimen for diabetic patients taking COCs. However, women with diabetes should be closely monitored while taking COCs.
The development of Crohn's disease and ulcerative colitis is associated with the use of COCs.
In rare cases, chloasma may develop, especially in women with a history of pregnancy spots. Women with a tendency to chloasma should avoid direct sunlight or ultraviolet radiation while taking COCs.
Women who develop severe depression while taking COCs should stop using these drugs and use alternative methods of contraception until a causal relationship of depressive symptoms with COC use has been assessed. Women with a history of major depressive episodes should be closely monitored and COCs should be discontinued if depressive symptoms recur.
Reduced efficiency.
The effectiveness of the COC may be reduced in case of skipping the pill, vomiting or diarrhea (see Section "Method of administration and doses") or through the use of concomitant medications (see Section "Interaction with other drugs and other types of interactions").
Reduced cycle control.
As with all COCs, irregular bleeding (spotting or breakthrough bleeding) may occur, especially during the first months of use. Therefore, evaluation of any irregular bleeding is only meaningful after the completion of an adaptation period of about three cycles.
If irregular bleeding persists or develops after previous regular cycles, it is recommended to use non-hormonal methods and carry out appropriate diagnostic measures to exclude a malignant neoplasm or pregnancy. These measures may include curettage.
Some women may not experience withdrawal bleeding after taking a break. If the COC was used in accordance with the section "Method of administration and doses", then pregnancy is unlikely. However, if the instructions in the section "Method of administration and dosage" before the first absence of withdrawal bleeding were not followed, or if there are no two withdrawal bleedings in a row, then pregnancy should be excluded before continuing to take COCs.
This medicinal product contains lactose and sucrose, therefore it should not be used in case of hereditary intolerance to galactose, lactose, glucose-galactose malabsorption or sucrose-isomaltase deficiency and fructose intolerance.
Use during pregnancy or lactation
When pregnancy is established, the drug should be stopped immediately.
If a woman becomes pregnant while taking the pills, further use should be stopped immediately.
The results of a large number of epidemiological studies have found neither an increased risk of birth defects in children born to women who used COCs before pregnancy, nor a teratogenic effect with inadvertent use of contraceptive pills in early pregnancy.
Breastfeeding. Hormonal contraceptives can reduce the secretion and composition of milk, and also pass into breast milk in small amounts, so taking these drugs during breastfeeding is contraindicated.
The ability to influence the reaction rate when driving vehicles or operating other mechanisms
The drug does not affect the ability to drive vehicles or other mechanisms.
Dosage and administration
Mode of application. Inside, in the order indicated on the package, at about the same time, one tablet a day, with a small amount of liquid.
Using the drug for the first time
Tri-Regol should be used from the 1st day of menstruation, 1 tablet per day for 21 days. Starting on days 2-7 is also possible, but during the first cycle it is recommended to additionally use a non-hormonal method of contraception (such as condoms or spermicides) during the first seven days of taking the pills.
Since the composition of tablets of different colors is different, pink tablets should be taken for the first 6 days, white tablets for the next 5 days, after which dark yellow tablets should be taken for 10 days. The sequence of taking tablets of different colors is indicated by numbers and arrows on the package.
After the end of the 21-day course of taking the drug, a 7-day break follows, during which menstrual bleeding usually occurs (usually on the 2nd or 3rd day). Regardless of whether bleeding has occurred or not and regardless of the duration, on the first day after a 7-day break, if further contraception is necessary, a 21-day course of Triregol should be started again. With regular intake of Tri-REGOL, the contraceptive effect is maintained even during a 7-day break.
According to the indicated scheme, Tri-Regol should be taken as long as prevention of pregnancy is desired.
Switching to Tri-Regol from another oral contraceptive: The first Tri-Regol tablet should be started the day after you took the last active (hormone-infused) pill from the blister pack of the previous contraceptive - no later than 1 day after the usual break in use previous combined hormonal contraceptive (or after taking the last placebo pill from the previous package).
Switching to Tri-Regol from a progestogen-only drug (low-dose oral contraceptive, injection, implant or intrauterine device): switching from low-dose oral contraceptives can be done on any day of the menstrual cycle (from the implant and intrauterine device the next day after their removal from the injection - on the day when the next injection should be scheduled). In this case, it is recommended to additionally use a barrier method of contraception during the first 7 days of taking the tablets.
After an abortion or after a miscarriage in the first trimester of pregnancy, the drug should be started immediately on the same day after the operation. Additional methods of contraception are not required.
After childbirth or abortion in the second trimester of pregnancy, the drug should be started in a woman who is not breastfeeding, 21-28 days after childbirth or abortion in the second trimester of pregnancy. If the start of oral contraception using the drug Tri-Regol occurs later, then it is necessary to additionally use a barrier method of contraception during the first 7 days of taking the tablets.
If sexual intercourse has already taken place, pregnancy should be excluded before taking the pills, or taking the pills should be postponed until the first menstrual bleeding.
Breast-feeding: For information on use while breast-feeding, see the Use During Pregnancy or Lactation section.
Missed pills: If a woman does not take a pill in time for any reason, she should take it within 12:00. In this case, there is no need to use additional methods of contraception. The remaining tablets should be taken at the usual time.
If more than 12:00 has passed, you must take the last missed tablet as soon as you remember it, even if you have to take two tablets on the same day. Then continue taking the drug as usual. In this case, additional non-hormonal methods of contraception (barrier methods, spermicides) must be used for the next 7 days.
If there are less than 7 tablets left in the current package, you should start taking the tablets from the next package immediately after taking the last tablet from the current package; this means that there should be no pause between packs. In this case, withdrawal bleeding is not expected until the end of the second pack; however, spotting and breakthrough bleeding may occur.
If withdrawal bleeding does not occur after completion of the second pack, pregnancy should be excluded until the tablets from the next pack are resumed.
Gastrointestinal diseases: in the presence of vomiting or diarrhea, the effectiveness of the drug decreases due to incomplete absorption of the active substances. Use additional non-hormonal methods of contraception (barrier methods, spermicides) while symptoms are present and for the next 7 days to prevent premature bleeding.
For vomiting or acute diarrhea that develops within 3-4 hours after taking a pill, see the tips described in the Missed pills section.
How to stop menstrual bleeding.
To delay menstrual bleeding, taking Tri-Regol tablets from a new package should be started with dark yellow tablets (last phase) the day after the end of the current package, without a pause between them. The duration of the delay in menstrual bleeding depends on the number of dark yellow tablets consumed from the second package. During this period, breakthrough bleeding or spotting may occur. Regular intake of Tri-Regol can be restored after the usual 7-day break.
often: vaginitis, candidiasis.
Benign, malignant and unspecified neoplasms (including cysts and polyps):
infrequently: breast cancer
very rarely hepatocellular carcinoma, liver adenoma.
From the immune system: rarely anaphylactic reactions, urticaria, angioedema, hypersensitivity reactions,
very rarely: exacerbation of systemic lupus erythematosus;
From the side of metabolism:
often: fluid retention;
infrequently: increased or decreased appetite
rarely: decreased glucose tolerance, hyperlipidemia, hypertriglyceridemia;
very rarely: exacerbation of porphyria. Mental disorders: often: mood changes, depression, depressive mood; infrequently: decreased or increased libido, nervousness.
From the nervous system: very often: headache, migraine
often: irritability, dizziness; very rarely: exacerbation of chorea, acute cerebrovascular accident. From the side of the organs of vision:
rarely contact lens intolerance very rarely optic neuritis*, retinal artery thrombosis, visual disturbance. On the part of the hearing organs: rarely otosclerosis. From the vascular system: infrequently - arterial hypertension,
rarely thrombosis, embolism
very rarely: worsening of varicose veins, myocardial infarction.
From the gastrointestinal tract: often: nausea, vomiting, pain in the stomach; infrequently stomach cramps, flatulence, diarrhea
very rarely pancreatitis.
From the side of the liver and choleretic tract: rarely cholestatic jaundice;
very rarely gallbladder disorder, cholelithiasis**.
From the skin and subcutaneous tissue: often: acne; infrequently: skin rash, urticaria, chloasma (melasma), hirsutism, alopecia,
rarely erythema nodosum, exudative erythema multiforme.
From the side of the kidneys and urinary tract:
very rarely hemolytic uremic syndrome.
From the reproductive system and mammary glands: very often breakthrough bleeding, spotting between menstruation;
often: chest pain, pain in the mammary glands, breast engorgement, secretion from the mammary glands, dysmenorrhea, menstrual changes, changes in erosion and secretion from the cervix, amenorrhea.
rarely vaginal discharge.
Study:
often: a change in the secret from the cervix, a decrease or increase in body weight.
rare: decrease in folate levels ***.
* Optic neuritis can lead to partial or complete loss of vision.
** The use of COCs may worsen current gallbladder disease and accelerate the development of this disease in women who have not previously experienced symptoms.
*** The use of COCs may lead to a decrease in serum folate levels.
The following adverse reactions (without indication of frequency) have been described by women who have used birth control pills:
From the side of metabolism and nutrition: hypercholesterolemia.
Mental disorders: irritability.
From the nervous system: cerebrovascular disorders.
From the vascular system: phlebitis.
From the skin and subcutaneous tissue: hypertrichosis, seborrhea.
From the skeletal muscles and connective tissue: a feeling of heaviness.
From the reproductive system and mammary glands: anovulatory cycles, oligomenorrhea, metrorrhagia, breast disorders. There have been reports of the following serious adverse reactions in women using COCs, detailed information on which is presented in the "Peculiarities of use" section:
- venous and arterial thromboembolic complications;
- arterial hypertension;
- liver tumors
- diseases that can develop or worsen when taking COCs, although evidence of this fact is inconclusive, include: Crohn's disease, ulcerative colitis, epilepsy, migraine, endometriosis, uterine fibroids, porphyria, systemic lupus erythematosus, herpes of pregnancy, chorea; hearing loss associated with otosclerosis; hemolytic-uremic syndrome, cholestatic jaundice;
- chloasma;
- acute and chronic hepatic disorders can lead to interruption of COC intake to normalize liver function.
The frequency of breast cancer diagnosis is slightly higher among PDA users. Since breast cancer is rarely diagnosed in women under 40 years of age, the overdiagnosis of breast cancer in women taking or recently taking COCs is small compared to the overall risk of developing breast cancer. A causal relationship with CPC has not been elucidated. More detailed information is indicated in the sections "Contraindications" and "Peculiarities of use".
Content
To prevent spermatozoa from fertilizing the egg during ovulation, gynecologists prescribe Tri-Regol oral contraceptive. The appointment of a medication occurs only after passing the tests and studying the individual data of the patient. Without them, you can not prescribe the drug, and in order to use the remedy correctly, read the instructions for use.
Instructions for use Tri-Regol
According to the pharmacological classification, Tri-Regol contraceptives are oral contraceptives of the three-phase type. Each phase of the tablets contains a hormone that inhibits sperm activity and prevents a woman from becoming pregnant. Strict adherence to the instructions with the rules of use will help to avoid unwanted pregnancy.
Composition and form of release
Since the drug is three-phase, the number of tablets inside the package is a multiple of three. Composition and description of each:
| Description | Pink, round biconvex tablets, white inside, glossy surface | white pills | Dark yellow shell |
| The concentration of ethinylestradiol, mcg per pc. | |||
| The concentration of levonorgestrel, mcg per pc. | |||
| Colloidal silicon dioxide, lactose monohydrate, magnesium stearate, corn starch, talc |
|||
| Shell Components | Sucrose, red iron oxide, talc, calcium carbonate, carmellose sodium, titanium dioxide, povidone, copovidone, colloidal silicon dioxide, macrogol | Same but without color | The same, but yellow iron oxide dye |
| Package | 6 pcs. in blister | ||
Pharmacodynamics and pharmacokinetics
The drug Tri-Regol refers to combined oral contraceptive estrogen-progestin agents that inhibit the pituitary secretion of a dose of hormones. It is necessary to consistently take pills with different contents of progestogen and estrogen. This ensures that the concentration of hormones in the blood is close to their level during a normal menstrual cycle. Due to this, a secretory change in the endometrium occurs.
The contraceptive effect of Tri-Regol is associated with the mechanism of action: levonorgestrel blocks the release of releasing factors of the luteinizing and follicle-stimulating hormones of the hypothalamus, inhibition of the secretion of gonadotropic hormones by the pituitary gland. This leads to inhibition (oppression) of the maturation and release of the egg (ovulation). Ethinyl estradiol keeps cervical mucus highly viscous, making it difficult for sperm to enter the uterine lining.
In addition to the contraceptive effect, Tri-Regol is able to normalize the menstrual cycle by replenishing the level of endogenous hormones with its constituent components. After 21 days of admission, a break is made for a week, during which menstrual bleeding occurs. Levonorgestrel is absorbed in four hours, excreted in 32 hours, binds to albumin, globulin.
Ethinylestradiol reaches its maximum concentration after 1-1.5 hours, is excreted in 52 hours. Metabolism of the substance occurs in the liver and intestines, it is found in the blood plasma within 12 hours after ingestion. Ethinylestradiol, by glucuronide conjugation, breaks down into active metabolites, which are excreted with the kidneys and intestines, similarly to levonorgestrel.
Indications for use
According to the instructions for use, Tri-Regol birth control pills have the only indication for use. It consists in oral hormonal contraception - the protection and protection of a woman from pregnancy. Tri-Regol can only be taken by women of childbearing age after being examined by a doctor and examining hormone and blood tests.
How to take Tri-Regol
The drug is taken at the same time every evening. Tablets are swallowed whole, not chewed, washed down with water. Every day for 21 days, it is taken on a tablet / day, then a week-long break is made for the onset of menstruation, then the reception is resumed. You can take Tri-Regol as long as you need contraception. When switching from another contraceptive to the drug, the regimen does not change.
After an abortion, taking the pills begins on the same or next day after the procedure. After childbirth, you can take the remedy only for those who are not breastfeeding. If you do not take the Tri-Regol tablet at the prescribed time, then you should drink it within the next 12 hours. If 36 hours have passed since the reception, contraception is not considered reliable. To avoid intermenstrual spotting, it is recommended to continue taking the already started package minus the missed one. To prevent pregnancy, it is better to use a barrier method of contraception.
drug interaction
When using Tri-Regol, you may experience side effects from combination with other drugs. Risky combinations:
- Ampicillin, Rifampicin, Chloramphenicol, Neomycin, antibiotics from the group of sulfonamides and tetracyclines, tranquilizers, Phenylbutazone weaken the effect of the drug;
- Indandione, any dose of an anticoagulant, coumarin derivatives, insulin, hypoglycemic drugs require adjustment of the dosage of the drug;
- Maprotiline, tricyclic antidepressants, beta-blockers increase the toxicity of the agent;
- the contraceptive reduces the effectiveness of Bromocriptine;
- Dantrolene increases the risk of hepatotoxicity, especially over the age of 35 years.
Side effects of Tri-Regol
Patients taking Tri-Regol report side effects. Common reactions include:
- breast engorgement, decreased libido;
- bleeding between periods, increased vaginal discharge, thrush;
- nausea, vomiting, jaundice, hepatitis;
- diarrhea, cholecystitis, headache, depression;
- increased frequency of epileptic seizures, eyelid edema, conjunctivitis;
- visual impairment, hearing loss;
- weight gain, increased blood glucose, decreased glucose tolerance;
- chloasma, skin rash, hair loss, skin itching;
- increased pressure, thrombosis, muscle cramps, coarsening of the voice.

Overdose
Instruction Tri-Regol warns that the symptoms of an overdose of the drug are nausea and uterine bleeding. The woman feels weak, dizzy, her blood pressure drops. In the first 2-3 hours after an overdose is detected, it is recommended to wash the stomach and give activated charcoal. There is no specific antidote.
Contraindications
Tri-Regol is prescribed with caution in compensated diabetes mellitus, varicose veins, multiple sclerosis, epilepsy, porphyria, bronchial asthma, uterine myoma, mastopathy, depression, tuberculosis. Contraindications for the use of the drug are:
- severe diseases, liver tumors;
- congenital syndromes of hyperbilirubinemia;
- cholecystitis, cholelithiasis, chronic colitis;
- decompensated heart disease, thromboembolism;
- phlebitis of the deep veins of the legs;
- hormone-dependent tumors or malignant neoplasms of the genital organs and mammary glands;
- familial form of hyperlipidemia, arterial hypertension;
- operations, prolonged immobilization, extensive injuries;
- pancreatitis, jaundice, severe diabetes mellitus;
- sickle cell or chronic hemolytic anemia, vaginal bleeding;
- migraine, cystic skid, otosclerosis, herpes of pregnant women;
- smoking over the age of 35, age over 40;
- lactose intolerance, any period of pregnancy (when it is detected, the intake is immediately stopped, because the risks of developmental disorders increase to a small extent), lactation;
- hypersensitivity to components.
Active ingredients:
I. One pink film-coated tablet contains ethinylestradiol 0.03 mg and levonorgestrel 0.05 mg
II. One white film-coated tablet contains 0.04 mg ethinylestradiol and 0.075 mg levonorgestrel
III. One dark yellow film-coated tablet contains 0.03 mg ethinylestradiol and 0.125 mg levonorgestrel.
Excipients:
Tablets I.
Sheath: Carmellose sodium, povidone, iron oxide red (E172), anhydrous colloidal silicon dioxide, macrogol 6000, copovidone, titanium dioxide (E171), calcium carbonate, talc, sucrose.
Tablets II.
Core: Silica colloidal anhydrous, magnesium stearate, talc, corn starch, lactose monohydrate (33.0 mg). ^ ^
Sheath: Carmellose sodium, povidone, anhydrous colloidal silicon dioxide, macrogol 6000, copovidone, titanium dioxide (E171), calcium carbonate, talc, sucrose. Tablets III.
Core: Silica colloidal anhydrous, magnesium stearate, talc, corn starch, lactose monohydrate (33.0 mg).
Sheath: Sodium carmellose, iron oxide yellow (E172), povidone, colloidal anhydrous silicon dioxide, macrogol 6000, copovidone, titanium dioxide (E171), calcium carbonate, talc, sucrose.
Description
Tablets I.
Pink, round biconvex film-coated tablets with glossy
surface
Tablets II.
White, round, biconvex film-coated tablets with glossy
surface
Tablets III.
Dark yellow, round, biconvex film-coated tablets with a glossy surface.
Indications for use
Tri-Regol is a hormonal contraceptive. Combined contraceptives act by inhibiting the action of gonadotropins. Although the primary mechanism of action of the drugs is the inhibition of ovulation, they also cause other changes, including changes in the consistency of cervical mucus, which makes it difficult for sperm to pass into the uterine cavity, as well as changes in the endometrium, which reduces the likelihood of implantation The drug should be taken only as directed and under regular medical supervision .
Contraindications
In the presence of pregnancy, severe liver disease, impaired fat metabolism, severe hypertension, severe diabetes mellitus and vaginal bleeding of unknown cause. The drug should also not be taken with previously transferred jaundice or herpes during pregnancy and with the following previously transferred or present conditions:
- diseases with the formation of blood clots (formation of a blood clot in the vessels) and with a predisposition to these diseases^,
- Liver tumor -
- a malignant tumor of the mammary glands or uterus.
Among women using hormonal contraceptives, the risk of certain diseases (eg, thromboembolic diseases, myocardial infarction, cerebral stroke) may increase. The risk of developing these diseases increases with age (over 35), especially among smokers. Therefore, women over 35 years of age are advised to completely stop smoking.
Pregnancy and lactation
If pregnancy is detected, the drug should be stopped immediately, because according to some studies, taking oral hormonal contraceptives in the early period of pregnancy slightly increases the risk of fetal developmental disorders.
Breast-feeding: Hormonal contraceptives can reduce lactation and change the composition of milk, and also pass into breast milk in small amounts, so the use of the drug during breast-feeding is not indicated.
Dosage and administration
The drug should be taken at the dose and for the time determined by the doctor. Taking the drug for the first time:
Use one film-coated tablet per day, preferably at the same time of day.
The drug should be started on the first day of the menstrual cycle and continued for 21 days. After this, it is necessary to take a seven-day break, during which menstrual-like bleeding occurs. The beginning and the correct sequence of taking the drug (first 6 pink, then 5 white and then 10 dark yellow tablets) are indicated by numbers and an arrow on the blister.
The next 21-day cycle of taking the drug should be started after a 7-day break. So each cycle starts on the same day of the week.
Switching to Tri-Regol after taking ANOTHER combination drug for 21 days:
The drug Tri-Regol should be taken according to the above scheme. The first Tri-Regol tablet should be taken on the first day after a seven-day break. If the previous contraceptive contained 22 tablets, the first tablet of Tri-Regol should be taken on the first day after a six-day break. If the previous contraceptive contained 28 tablets, the first tablet of Tri-Regol should be taken without interruption. §*”
v S *
Switching to taking the drug Tri-Regol after taking the drug "mini", which contains a progestogen
The first Tri-Regol tablet should be taken on the first day of menstruation, even if the mini tablet has already been taken. In this case, there is no need to use an additional method of contraception.
In the presence of vomiting or diarrhea, it is necessary to additionally use another (non-hormonal) method of contraception.
With the appearance of intermenstrual bleeding, you should continue taking the drug, since bleeding usually stops spontaneously. If the bleeding does not stop or recurs, you should consult a doctor.
If no bleeding occurs during the 7-day break, pregnancy should be excluded.
After childbirth or after an abortion, the course of administration can be started as directed by the doctor, but not earlier than the first day of the second menstruation.
If, for medical reasons, an earlier start of contraception is justified, then the drug should be started on the first day of the first menstruation, but in the first two weeks it must be combined with another (non-hormonal) method of contraception.
Side effect
Triregol, like all medicines, can cause side effects.
While taking the drug, nausea, vomiting, headache, tension of the mammary glands, changes in body weight and libido, depressed mood, chloasma (pigment spots), discomfort when wearing contact lenses, intermenstrual bleeding, increased blood pressure, increased blood glucose , skin rash, the appearance of a condition with the formation of blood clots (thrombosis), diseases of the liver and gallbladder, fatigue, diarrhea.
Inform your doctor about the appearance of the above complaints or symptoms.
Overdose
If you have taken more than the prescribed dose of the drug, contact your doctor immediately.
Children who accidentally take large doses of oral contraceptives do not have severe abnormalities, so there is no need for drug treatment for overdose. If an overdose of the drug is detected within 2-3 hours, a gastric lavage should be performed. There is no specific antidote, treatment is symptomatic.
Interaction with other drugs
Be sure to tell your doctor or pharmacist if you are taking or have recently taken any medicines, including medicines sold without a doctor's prescription.
The drug should be used with caution simultaneously with:
- ampicillin, rifampicin, chloramphenicol, neomycin, penicillin B, sulfonamides, tetracyclines, dihydroergotamine, tranquilizers, phenylbutazone (the contraceptive effect may decrease, therefore it is necessary to use another, non-hormonal contraceptive method),
- anticoagulants, coumarin or indandione derivatives (it is necessary to re-determine the prothrombin time and, if necessary, change the dose of the anticoagulant),
- tricyclic antidepressants, maprotiline, beta-blockers (their bioavailability and toxicity may increase),
- oral antidiabetic agents, insulin (it may be necessary to change the dose of these agents),
- bromocriptine (decreased effectiveness),
- hepatotoxic drugs, especially with dantrolene (the risk of hepatotoxicity increases, especially in women over 35 years of age).